2272
The Role of the Human Visual Cortex in Assessment of the Long-term Durability of Retinal Gene Therapy in Follow-on RPE65 Clinical Trial Patients1Center for Advanced Retinal and Ocular Therapeutics (CAROT), University of Pennsylvania, Philadelphia, PA, United States, 2F.M. Kirby Center for Molecular Ophthalmology, Scheie Eye Institute, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 4University of Pittsburgh, Pittsburgh, PA, United States, 5Center for Cellular and Molecular Therapeutics, The Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 6Westat Biostatistics and Data Management Core, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Leber’s congenital amaurosis (LCA) is a rare blinding disease with no cure. Recently, patients with LCA underwent retinal gene therapy and regained their vision to a great extent. We followed this group of LCA patients before and up to three years on an annual basis after gene therapy using fMRI to assess the feasibility and durability of retinal gene therapy over time and the role fMRI could play as an outcome measure for other future retinal interventions.
Study Goal
To evaluate the efficacy, feasibility, and durability of retinal gene therapy over time using fMRI and to identify the role fMRI may play as an outcome measure in the evaluation of patients who regain their sight through retinal gene or cell intervention.Background
Leber’s congenital amaurosis (LCA) is a rare blinding disease caused by more than a dozen different gene mutations and it is usually inherited in an autosomal recessive fashion. It is symptomatic at birth or in the first few months of life and affects around 1 in 81,000 people. LCA patients with an RPE65 gene mutation are good candidates for gene therapy as the degeneration of retinal cells is slow, providing an extended potential time window for intervention. Recent encouraging results from a Phase I clinical trial where a group of RPE65 patients received gene therapy to their worse-seeing eye resulted in continuation to a second clinical trial where the same patients received a subretinal injection of the RPE65 gene to their contralateral eye. Having access to these patients before and after gene therapy, using the standard checkerboard (8Hz) stimuli, fMRI was performed at baseline and follow-up on an annual basis for three years testing the newly injected eye.Material and Method
MRI scans were conducted on a 3T system using a 32-channel head coil and BOLD sequence. The fMRI paradigm programmed in E-Prime, consisted of 15-second blocks of flickering (8-Hz) black and white checkerboards, which consisted of three contrasts of high, medium, and low, interleaved with 15 seconds of blank (black) screens. fMRI stimuli were delivered using Resonance Technology VisuaStim video goggles. Subjects were monitored for motion using the scanner's real time imaging feature and the image acquisitions were repeated for translational and rotational movements greater than 0.8 mm. All fMRI analyses were performed using the general linear model (GLM) and the contrast of active blocks (checkerboard stimuli) minus the rest blocks (blank, black screen) as implemented in BrainVoyagerQX.Results
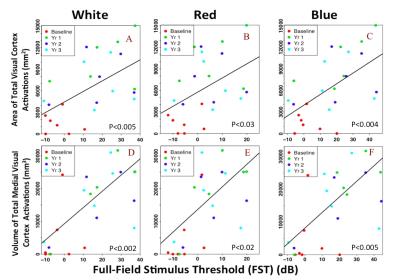
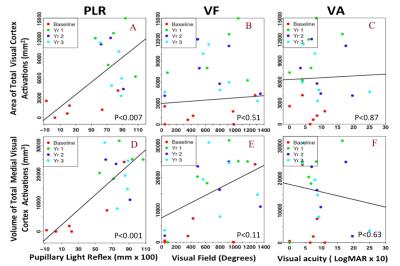
As depicted in Figure 1, longitudinal fMRI results from LCA patients showed minimal or no cortical responses at baseline (column 1) except for one subject (NP15). All subjects showed increased cortical activations (columns 2-4) that lasted for three years the delivery of gene therapy to the untreated eye (contralateral eye). Increased activation over time was observed for the entire visual cortex, particularly for the primary visual areas. Linear mixed effect models for longitudinal data were performed to assess the association between the fMRI results and clinical measures. As presented in Figures 2 and 3, fMRI statistical results showed significant association for the primary and overall visual cortex activations with several clinical measures used as outcome measures, including full field light sensitivity threshold (FST) for white, red, and blue colors, visual field (VF), and pupillary light reflex (PLR).Conclusion
In agreement with our previous reports (1,2), despite severe and long-term visual impairment, RPE65 subjects showed intact visual pathways that became responsive and strengthened after retinal gene therapy. fMRI results not only attest to the efficacy and feasibility of this revolutionary treatment but also reveal the long-lasting effect with only a one-time subretinal delivery of the AAV-mediated retinal gene therapy for subjects with autosomal recessive mutations in RPE65 mutation. Most importantly, the fMRI results significantly correlated with patients’ clinical outcome and paralleled the results recently reported for the long-term clinical evaluations of the same subjects (3). Results from this study demonstrate that fMRI could play an important role in providing complementary information to patients’ ophthalmic clinical evaluation and may have utility to be considered as an outcome measure for other future retinal interventions.Acknowledgements
This study was supported by R01EY025287-01A1, R21EY020662 from the National Eye Institute. This study was also funded in part by the Foundation Fighting Blindness–sponsored CHOP-PENN Pediatric Center for Retinal Degenerations, Clinical Translational Science Award NIH/NCRR UL1-RR-024134, 1R01EY019014-01A2, Research to Prevent Blindness, the Paul and Evanina Mackall Foundation Trust at Scheie Eye Institute and the F. M. Kirby Foundation.References
1- Ashtari M, Cyckowski LL, Monroe JF, Marshall KA, Chung DC, Auricchio A, et al. The human visual cortex responds to gene therapy-mediated recovery of retinal function. J Clin Invest. 2011;121(6):2160-8. doi: 10.1172/JCI57377. PubMed PMID: 21606598; PubMed Central PMCID: PMC3104779.
2- Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4(120):120ra15. Epub 2012/02/11. doi: 10.1126/scitranslmed.3002865. PubMed PMID: 22323828.
3- Bennett J, Wellman J, Marshall KA, McCague S, Ashtari M, DiStefano-Pappas J, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388(10045):661-72. doi: 10.1016/S0140-6736(16)30371-3. PubMed PMID: 27375040.