2269
Age-related MRI changes in the connective tissues of the eyeYolandi van der Merwe1,2, John S. Gnalian3, Ning-Jiun Jan1,2, Ian A. Sigal1,2, and Kevin C. Chan1,2,3
1Department of Ophthalmology, University of Pittsburgh, Pittsburgh, PA, United States, 2Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, United States, 3Neuroimaging Laboratory, University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
The structural organization and compositions of the corneoscleral shell determine the biomechanical behavior of the eye, and are important in aging and diseases such as glaucoma and myopia. However, characterizing the structure and composition of the eye and their changes with age or intraocular pressure remains a challenge. In this study, we showed that T2 mapping, magnetization transfer MRI and diffusion tensor MRI can be used to detect and differentiate age- and intraocular pressure-related changes in the porcine eyes. Multi-modal MRI may be useful for evaluating the biomechanical and (patho-)physiological mechanisms in the corneoscleral shell non-invasively and quantitatively.
Purpose
The sclera and cornea are dense and fibrous connective tissues that form the outer coat of the eye for maintaining shape and protecting delicate tissues like the retina. These load-bearing connective tissues can dynamically interact with changing physiological conditions1,2. The extracellular matrix in the corneoscleral shell may also be remodeled in aging3-5, glaucoma6, and myopia7, and plays an important role in transcorneal and transscleral drug delivery8,9. To date, there are limited non-invasive methods to assess the relationships between ocular integrity, intraocular pressure (IOP), neuropathological events and functional outcomes in aging and glaucoma. Although several techniques can help characterize the microarchitecture and biochemistry of excised ocular tissue sections10, they do not allow non-destructive and multi-modality assessments of the same sclera and cornea tissues in the intact globe11. MRI offers non-invasive and multi-parametric methods for assessing the impaired visual system without depth limitation. However, MRI studies of the corneoscleral shell have been limited, partly due to the intrinsically fast transverse magnetic resonance relaxation of the fibrous tissues and the resulting low MRI signal intensities available for biological examinations. Recently, our group demonstrated the use of the magic-angle enhancement effect to improve MRI sensitivity for detecting the structural details of the collagen-rich sclera and cornea tissues, and their changes in T2 and T2* transverse relaxation times in tissues fixed at various levels of IOP12. In this study, we aim to non-invasively understand the structural organization and compositions of the corneoscleral shell in the intact eyes under aging and glaucomatous conditions using multi-modal MRI.Methods
Twenty 3-months-old (mos) and nineteen 9 mos porcine eyes were fixed at low IOP at 0-5 mmHg (n=12 at 3 mos; 9 at 9 mos), moderate IOP at 20-30 mmHg (n=4 at 3 mos; 4 at 9 mos), and high IOP at 40-50 mmHg (n=4 at 3 mos; 6 at 9 mos) using a gravity perfusion fixation system and a cannula inserted into the anterior chamber12. All porcine eye experiments were performed using a 9.4-Tesla Varian/Agilent MRI scanner with a 38 mm-diameter transmit-receive volume coil and the following imaging parameters. i) T2 mapping: TR/TE=1000/9.47ms, EST=9.47ms, number of echoes=5; ii) magnetization transfer MRI (MTI): 9.5µT saturation pulses at 4000Hz off-resonance and 150ms pulse length, TR/TE=1500/16.9ms; iii) Diffusion tensor MRI (DTI): 2 non-diffusion-weighted images and 12 diffusion gradient directions at b=420s/mm2, TR/TE=2300/22ms. All T2 mapping, MTI and DTI shared the same slice geometry, with in-plane resolution=125×125µm2 and slice thickness=1mm. Hydrogel was applied to keep the surface of the eyes moist throughout the experiment. Multi-modal MRI parameters were measured near the magic angle at about 55o to the main magnetic field (Bo), and compared across age and IOP using 2-way ANOVA.Results
Figure 1 shows the multi-modal MRI of the central slice of a representative older porcine eye at high intraocular pressure. When measuring near the magic angle (colored arrows in Figure 1), quantitative comparisons in Figure 2 showed significant IOP effect in T2 of cornea (ANOVA, p<0.001), whereas significant age effect was observed in magnetization transfer ratio (MTR) of both sclera (ANOVA, p<0.01) and cornea (ANOVA, p<0.01). Significant age effect was also observed in axial, radial and mean diffusivities of the cornea in DTI in Figure 3 (ANOVA, p<0.05).Discussion
While the baseline MTR in fibrous tissues may be primarily due to tissue collagen concentration, changes in MTR may be due to (patho-)physiological changes in crosslinking state and glycosaminoglycan concentration13. The age-related changes in MTR of both sclera and cornea may be partly due to microscopic structural remodeling such as crosslinking in the collagen-rich tissues14. Aging and crosslinking may also reduce diffusion in collagen tissues leading to the observed diffusivity changes in DTI of the cornea in older eyes15,16. Based on the current results, MTI and DTI appeared to be more sensitive than T2 mapping in detecting age-related changes in the eye. Multi-modal ocular MRI detection of structural and compositional tissue changes may offer a translational imaging means that allows non-destructive measurements of connective tissue properties in the eye, and may be used as a non-invasive tool to guide vision preservation in age-related vision diseases.Conclusions
This work demonstrates the sensitivity and specificity of multi-modal MRI on age- and IOP-related changes in the porcine eyes. Multi-modal MRI may be useful for evaluating the biomechanical and (patho-)physiological mechanisms in the corneoscleral shell non-invasively and quantitatively.Acknowledgements
This work was supported by the National Institutes of Health P30-EY008098, R01-EY023966, R01-EY025011 and UL1-TR000005 (Bethesda, Maryland); BrightFocus Foundation G2013077 (Clarksburg, Maryland); Stimulating Pittsburgh Research in Geroscience Pilot Project Program Award (Pittsburgh, PA); Eye and Ear Foundation (Pittsburgh, Pennsylvania); and Research to Prevent Blindness (New York, New York).References
[1] Liu J, He X. Corneal stiffness affects IOP elevation during rapid volume change in the eye. Invest Ophthalmol Vis Sci 2009;50(5):2224-2229. [2] Kimball EC, Nguyen C, Steinhart MR, et al. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp Eye Res 2014;128:129-140. [3] Coudrillier B, Pijanka J, Jefferys J, et al. Collagen structure and mechanical properties of the human sclera: analysis for the effects of age. Journal of biomechanical engineering 2015;137(4):041006. [4] Steinhart MR, Cone-Kimball E, Nguyen C, et al. Susceptibility to glaucoma damage related to age and connective tissue mutations in mice. Exp Eye Res 2014;119:54-60. [5] Grytz R, Fazio MA, Libertiaux V, et al. Age- and race-related differences in human scleral material properties. Invest Ophthalmol Vis Sci 2014;55(12):8163-8172. [6] Coudrillier B, Pijanka JK, Jefferys JL, Goel A, Quigley HA, Boote C, Nguyen TD. Glaucoma-related Changes in the Mechanical Properties and Collagen Micro-architecture of the Human Sclera. PLoS One. 2015 Jul 10;10(7):e0131396. [7] Rada JA, Shelton S, Norton TT. The sclera and myopia. Experimental eye research 2006;82(2):185-200. [8] Molokhia SA, Jeong EK, Higuchi WI, Li SK. Transscleral iontophoretic and intravitreal delivery of a macromolecule: study of ocular distribution in vivo and postmortem with MRI. Exp Eye Res 2009;88(3):418-425. [9] Cassagne M, Laurent C, Rodrigues M, et al. Iontophoresis transcorneal delivery technique for transepithelial corneal collagen crosslinking with riboflavin in a rabbit model. Invest Ophthalmol Vis Sci 2014. [10] Nguyen TD, Ethier CR. Biomechanical assessment in models of glaucomatous optic neuropathy. Exp Eye Res 2015. [11] Ko MW, Leung LK, Lam DC, Leung CK. Characterization of corneal tangent modulus in vivo. Acta ophthalmologica 2013;91(4):e263-269. [12] Ho LC, Sigal IA, Jan NJ, et al. Magic angle-enhanced MRI of fibrous microstructures in sclera and cornea with and without intraocular pressure loading. Invest Ophthalmol Vis Sci 2014;55(9):5662-5672. [13] Laurent D, Wasvary J, Yin J, Rudin M, Pellas TC, O'Byrne E. Quantitative and qualitative assessment of articular cartilage in the goat knee with magnetization transfer imaging. Magn Reson Imaging 2001;19(10):1279-1286. [14] Gautieri A, Passini FS, Silvan U, et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol 2016. [15] Kokkonen HT, Makela J, Kulmala KA, et al. Computed tomography detects changes in contrast agent diffusion after collagen cross-linking typical to natural aging of articular cartilage. Osteoarthritis Cartilage 2011;19(10):1190-1198. [16] Carpenter DG. Biological aging as diffusion phenomenon. Bull Math Biophys 1969;31(3):487-504.Figures
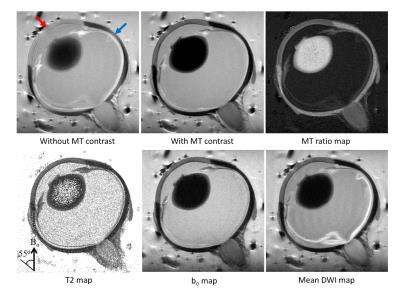
Figure 1. Multi-modal
MRI of the central slice of a
representative older porcine eye at high intraocular pressure. Top row shows
the magnetization transfer MRI (MTI) of the whole porcine eye before (left) and
after applying magnetization transfer (MT) signal suppression (middle), as well
as the MT ratio map (right). Bottom row shows the T2 map,
non-diffusion-weighted (bo) map, and mean diffusion weighted MRI
(DWI) map. Multi-modal MRI parameters were measured near the magic angle at
about 55o to the main magnetic field (Bo) (Red arrow for
cornea; blue arrow for sclera),
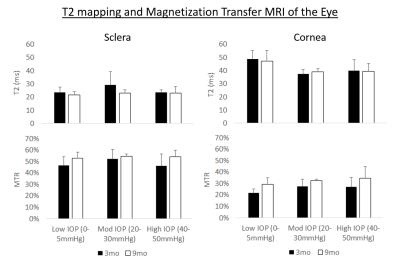
Figure 2. Quantitative
comparisons (mean ± standard deviation) of T2 (top
row) and magnetization transfer ratio (MTR) (bottom row) in the sclera (left
column) and cornea (right column). Significant intraocular pressure (IOP)
effect was observed in T2 of cornea (ANOVA, p<0.001), whereas significant
age effect was observed in MTR of both sclera (ANOVA, p<0.01) and cornea
(ANOVA, p<0.01).
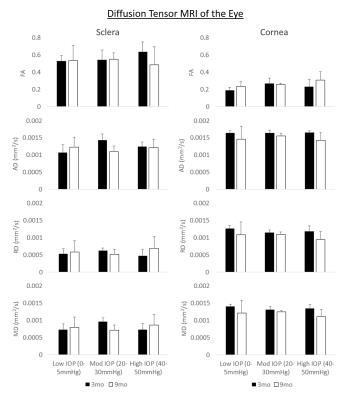
Figure 3. Quantitative
diffusion tensor MRI comparisons (mean ± standard
deviation) of fractional anisotropy (FA, top row), axial
diffusivity (AD, second row), radial diffusivity (RD, third row), and mean
diffusivity (MD, bottom row) in the sclera (left column) and cornea (right
column). Significant age effect was observed in AD, RD and MD of the cornea
(ANOVA, p<0.05).