2266
Morphometric Analyses of Visual Cortex in Patients with Retinitis Pigmentosa1Radiology, Zhengzhou University People's Hospital (Henan Provincial People's Hospital), Zhengzhou, People's Republic of China, 2Ophthalmology, Zhengzhou University People's Hospital (Henan Provincial People's Hospital), Zhengzhou, People's Republic of China, 3GE Healthcare MR Research China, People's Republic of China
Synopsis
Possible effects on the structures of visual cortex using high-resolution anatomical MRI have not been established so far. The aim of this study was to investigate volumetric alterations in visual cortex of RP patients using voxel based morphometry (VBM). Our results demonstrated that RP is associated with degeneration of structures in the visual cortex, and prevention of visual cortical degeneration may need to become a new therapeutic goal for RP patients, which may have significant meaning for clinical to guide RP patients' treatment in a more reasonable way in future.
BACKGROUND AND PURPOSE
Previous reports using DTI have shown the damage in optic nerve and optic radiation of patients with retinitis pigmentosa(RP), but possible effects on the structures of visual cortex using high-resolution anatomical MRI have not been established. In this study, our aim was to investigate volumetric alterations in visual cortex of RP patients using voxel based morphometry(VBM).MATERIALS AND METHODS
Twenty-nine patients (17men,12women;mean age,37years;range,16–63years) and twenty-seven healthy controls (17men,10women;mean age,38years;range,18–61years) were enrolled in this study. Measures of visual field (VF) and visual acuity (VA) were performed on all subjects. In addition, a 1 mm isotropic resolution anatomic 3D T1-weighted acquisition based on magnetization-prepared fast spoiled gradient echo sequence (Discovery MR 750, GE Healthcare) was acquired for each participant, with the following parameters: TR/TE=8.2/3.2ms, FOV=25.6×25.6cm, matrix=256×256, slice thickness=1mm, no gap, slices=180, NEX=1. VBM was used to analyze the difference in cortical grey matter volume between patients and controls. Morphometric values in terms of volume were extracted from ROIs situated in broadmann area 17(primary visual cortex, V1), 18(secondary visual cortex, V2), and 19(other associative visual cortex, OAVC). Further analyses were performed to determine the correlation of the grey matter volume in V1 and V2 of RP patients with the mean deviation of visual field (MDVF) and mean VA, respectively.RESULTS
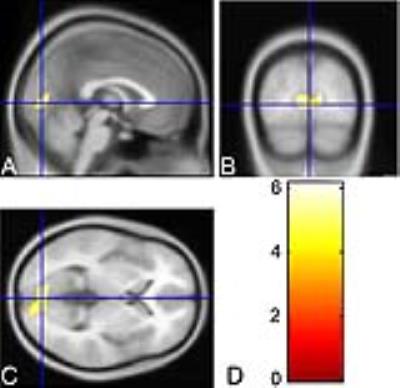
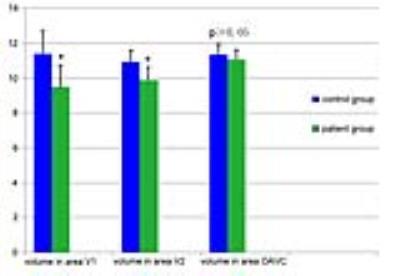
Compared with healthy controls, in patients with RP volumetric reduction in the middle part of medial occipital cortex was found (P<0.01,FDR corrected)(Fig 1.). RP patients showed significantly lower volume in V1 and V2 as compared to control subjects (P<0.001); however, no significant difference in OAVC volume between patients and controls was seen (P>0.05)(Fig 2.). In addition, for RP patients, the grey matter volume in V2 was significantly correlated to mean VA (r=-0.408,P=0.028) and mean MDVF (r=0.386, P=0.038), while that in V1 was only significantly correlated to mean VA values (r=-0.408,P=0.028)(Fig 3.).DISCUSSION
In this study, our main finding showed the volumetric loss of visual cortex in RP. A similar loss has previously been reported in glaucoma.[1] In glaucoma, it is rather intuitive that the retrobulbar visual pathway may be involved in its pathology as there is a loss of retinal ganglion cells (RGCs) and their axons form the optic nerve. However, in RP, the primary loss of vision is due to damage of the photoreceptors,[2] which are first-order neurons. It is reported that gradual loss of RGCs (third-order neurons) in RP patients takes place with the progressive death of photoreceptor cells, governed by the trans-synaptic neuronal degeneration mechanism,[3] and may lead to the damage in optic nerves[4] and optic radiation[5] of patients with RP. This may also explain the volumetric reduction of the visual cortex in RP patients.
V1 is the simplest, earliest cortical visual area to process visual stimuli, while V2 is the first region within the visual association area, which receives strong feedforward connections from V1 and sends strong connections to V3, V4, and V5. As VA of RP patients decreased gradually and visual input becomes more and more less, visual cortex can't be stimulated enough and then cortical deprivation may happen in RP . VA may reflect visual deprivation in RP. The significant correlations of VA loss with volumetric reduction in V1 and V2 respectively for RP patients suggest that the reduced volume in V1 and V2 is of functional relevance and may reflect visual deprivation in RP.
VF loss in RP has been attributed to the death of photoreceptors.[6] With the death of photoreceptor cells and following loss of RGCs, the injury in visual cortex of RP advances. VF defects may reflect the pathology of visual cortex degeneration in RP. As the damage along the visual pathway of RP is caused by a transneuronal degeneration mechanism,[3] the volumetric loss of V1 rather than V2 might be the leading consequence of visual cortex degeneration in RP. However, our study showed that there was a significant correlation of V2 volume with VF, but no correlation of V1 volume with VF in RP. In general, in RP, there is a delay between pathological changes and clinically evident symptoms.[7] Consequently, RP patients diagnosed by typical symptoms in our study were likely manifesting a chronic stage of visual cortex damage. Changes of V1 volume in RP might become less informative as pathological conditions of the visual cortex progress from acute to chronic, which may explain the reason why no correlation of V1 volume with VF was seen in current study.
CONCLUSIONS
RP is associated with degeneration of structures in the visual cortex. Prevention of visual cortical degeneration may need to become a new therapeutic goal for RP patients.Acknowledgements
We thank Dr. Zhonglin Li, Department of radiology, Henan Provincial People's Hospital, for technical guidance, and Dr. Shuyin li, Department of ophthalmology, Henan Provincial People's Hospital for clinical assistance. This study is supported by the National Natural Science Foundation of China under Grant (No.81271534, Zhengzhou, China).References
[1] Hernowo AT, Boucard CC, Jansonius NM, et al. Automated morphometry of the visual pathway in primary open-angle glaucoma. Investigative Ophthalmology & Visual Science, 52(5): 2758e2766, 2011.
[2] Hamel CP. Gene discovery and prevalence in inherited retinal dystrophies[J]. C R Biol, 2014, 337 (3): 160-6.
[3] Gartner S, Henkind P. Pathology of retinitis pigmentosa. Ophthalmology 1982;89:1425-32.
[4] Zhang Y, Guo X, Wang M, et al. Reduced Field-of-View Diffusion Tensor Imaging of the Optic Nerve in Retinitis Pigmentosa at 3T. AJNR Am J Neuroradiol. 2016;37(8):1510-5.
[5] Naonori Ohno, Hideki Murai, Yukihisa Suzuki, et al. Alteration of the optic radiations using diffusion tensor MRI in patients with retinitis pigmentosa. Br J Ophthalmol 2015;99:1051-4.
[6] Walia S, Fishman GA, Edward DP, et al. Retinal nerve fiber layer defects in RP patients. Invest Ophthalmol Vis Sci 2007;48:4748-52.
[7] Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet 2006;368:1795-809.
Figures