2264
Role of visual cortex during after and before critical developmental period in early blind and late blind: An fMRI study1Department of NMR and MRI Facility, All India Institute of Medical Sciences, New Delhi, India, 2Dr. RP Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India
Synopsis
Visual cortex is preserved and is performing during non-visual stimuli showing development of specific compensatory mechanisms associated with visual area in blind subjects. Blind people process auditory language stimuli faster than sighted people. Some language functions suggest cortical reorganization in the blind subjects.
Purpose and Background
Blind people often localize and identify sounds more accurately than sighted controls using specific auditory task(s), however the mechanism and cause of cross-modal plasticity before and after critical developmental period remains unclear [1]. We hypothesized that specialized visual areas, once established through visual experience, assist in other tasks, eg. discrimination of semantic versus ambient sounds, in early and late blind subjects.Objective
To study the functional changes associated with sensory deprivation during developmental period, by assessing the phonological perception in young and adolescent groups of early and late blind subjects.Methods
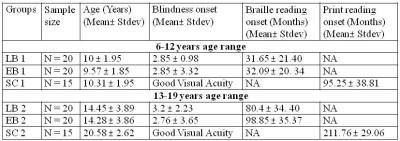
Early blind (EB) and late blind (LB) subjects and sighted controls (SC) (all right handed) in two age groups 6-12 years and 13-19 years were recruited from the clinics of our institute (Table 1). Standard diagnostic and exclusion criteria were followed. Functional MRI scans were carried out using 3T MR scanner (Achieva 3.0T TX, Philips, Netherlands). Auditory task for assessment of orientation and semantic components of language consisted of four parts: (a) words for which subject was instructed to speak the antonyms, (b) words for which the subject had to speak the synonyms, (c) orientation based questions to estimate and correlate the spatial orientation referred by the auditory cue with respect to the position and posture of the subject (supine position inside the MRI scanner), and (d) animal and vehicle sound recognition task in which auditory recordings of vehicles and animals was given and from which the subject had to identify the sound and speak out. Auditory cue were presented and generated with the help of E-prime and MRI compatible headphone inside MR scanner. Single-shot EPI sequence was used for the BOLD study in the whole brain, with number of slices: 30, slice thickness 4.5 mm; TR: 2000 ms, TE: 30 ms, FOV: 230 mm, flip angle: 90º, number of dynamics: 210. Pre- and post-processing were carried out using SPM8 [2]. The BOLD clusters were converted from MNI template to the Talairach and Tornoux coordinates, for estimation of anatomical areas [3]. One way ANOVA (p<0.001, cluster threshold 10) was used for intra- and inter-group analyses. Pearson correlation between BOLD activation, age onset and blindness duration onset were assessed using SPSS (version 16, SPSS Inc., IBM Business Analytics).Results
During phonological processing, bilateral cerebellum and frontal area, Broca's area and Wernicke's area activation was observed in SC1, and left lateralized in Broca's and Wernicke's area in SC2 group (Figure 1). In LB1 group phonological performances were actively supported by secondary visual cortical and primary auditory areas, but in LB2, primary visual cortical area along with Broca’s, Wernicke’s areas and primary auditory cortex were actively responding. In EB1, bilateral activation was observed in striate, extrastriate areas, Wernicke's area along with frontal activation while in EB2 bilateral activation was observed in striate, extrastriate areas along with Broca's and Wernicke's area in association with frontal areas.Discussion
The three (EB1, EB2, LB2) groups had significantly different response modulations in bilateral parts of cortex surrounding primary visual cortex (V1) and higher visual areas. Activation in striate and extrastriate varied as a role of semantic or syntactic content during speech processing [4]. Occipital responses to all acoustic cues increased in blind children with age, as revealed (Figure 2) by Pearson’s correlation coefficient [5]. Occipital cortex of blind individuals is functionally coupled with executive control areas of frontal cortex [6]. Selective processing of sound locations in EB and LB groups was associated with greater bilateral activation in posterior temporal areas, and inferior and superior parietal lobes [7]. Bilateral activity was observed in primary and secondary auditory cortices, inferior and superior parietal cortices, and superior and inferior frontal gyri showing dorsal stream is dominantly performing in EB2 group and LB2 group for processing of semantic words. The spectral contour of the auditory processing in both late blind and early blind group involves lingual gyrus in occipital ventral stream [8]. In sighted controls, fusiform gyrus and inferior temporal gyrus activation may be ascribed to during complex visual tasks such as attention to shapes and visual processing of objects [4].Acknowledgements
No acknowledgement found.References
1. Sadato et al Critical period for cross-modal plasticity in blind humans: a functional MRI study. NeuroImage 2002; 16: 389–400
2. Friston KJ, Holmes Statistical parametric maps in functional imaging: A general linear approach, Hum. Brain Mapp. 1994; 2:189-210
3. Talairach J, and Tornoux P Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging 1988; Stuttgart: thieme
4. Burton H et al. Recognition Memory for Braille or Spoken Words: An fMRI study in Early Blind, Brain Res. 2012; 1438:22-34
5. Garg A, et al. Orienting auditory spatial attention engages frontal eye fields and medial occipital cortex in congenitally blind humans. Neuropsychologia. 2007;45:2307–2321
6. Deen B, Saxe R, Bedny M. Occipital cortex of blind individuals is functionally coupled with executive control areas of frontal cortex, J Cogn Neurosci. 2015;27:1633-47.
7. Voss P et al Relevance of Spectral Cues for Auditory Spatial Processing in the Occipital Cortex of the BlindFront Psychol. 2011; 2: 48.
8. Gougoux F, A Functional Neuroimaging Study of Sound Localization: Visual Cortex Activity Predicts Performance in Early-Blind Individuals, PLoS Biol. 2005; 3: e27.
Figures