2256
Relationship Between DTI of the Brainstem Auditory Pathway and Latency of the Auditory M100 Response is Altered in Autism Spectrum Disorder1Radiology, Children's Hospital of Philadelphia, Philadelphia, PA, United States, 2Radiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Alterations to the auditory system’s structure and function may underlie the auditory processing and language disorders prevalent in autism spectrum disorder (ASD). This multimodal study compared DTI of the brainstem auditory pathway to magnetoencephalography (MEG) measures of auditory conduction velocity (M100 latency). The M100 latency measures the time between auditory stimulus and auditory cortex response. DTI and MEG were acquired from 29 children with ASD and 31 controls. Increased brainstem auditory pathway FA was predictive of faster signal conduction in controls (shorter M100 latency) (p<0.01), but not ASD. These results indicate ASD impacts the structure-function relationships throughout the auditory system.
Introduction
Efficient conduction of acoustic information from the ear to the auditory cortex requires white matter capable of conveying action potentials along the entire length of the auditory pathway. In autism spectrum disorder (ASD), the white matter of the auditory radiation and superior temporal gyrus undergo abnormal maturation and functional lateralization [1, 2]. In addition, the 100ms (M100) auditory response at the superior temporal gyrus, as measured with magnetoencephalography (MEG), is delayed in ASD, indicating abnormally slow conduction velocity and processing [3]. Previous studies investigating the role of cerebral auditory white matter microstructure in modulating the M100 latency have observed a decoupling of the structure-function relationship in ASD[4, 5]. Such alterations may comprise the biological basis for auditory processing and language disorders prevalent in ASD. However, the M100 latency integrates conduction and processing speed along the entire length of the auditory system from the ear to auditory cortex. Indeed, delayed early auditory brainstem responses have been observed in ASD [6]. This study extends prior findings by combining diffusion MR measures of brainstem white-matter microstructure with MEG measures of M100 latency with the hypothesis that the structure-function relationship is altered in ASD.Methods
This multimodal study included 29 children with ASD (mean age= 10.5±2.3years) and 31 age matched typically developing controls (mean age= 10.5±2.7years) with diffusion MRI and MEG data. DTI was acquired on a 3T Verio (Siemens) with 30 diffusion gradient directions at b=1000 s/mm2, one b=0 s/mm2 volume, TR/TE= 11s/76ms, voxel size 2x2x2mm, and 128x128 matrix. Probabilistic tractography (FMRIB's Diffusion Toolbox) was used to define the left and right brainstem auditory pathway from the superior olivary complex to the medial geniculate nucleus (figure 1). Fractional anisotropy (FA), mean diffusivity (MD) and radial diffusivity (RD) were measured voxelwise in left and right auditory pathways. MEG was performed with a whole head system (CTF). Tones at 200, 300, 500, and 1000Hz of 300ms duration were presented with 130 trials per condition. The M100 response for each frequency and hemisphere (8 total conditions) was source localized to the left and right superior temporal gyri and the M100 peak latency determined. For unimodal analysis, DTI measures and M100 latency were separately modeled with effects of hemisphere, group, age, and their interaction terms. For the multimodal analysis, M100 was modeled with group, hemisphere, tone frequency, DTI measure, and their interactions as fixed effects and subject as a random effect.Results
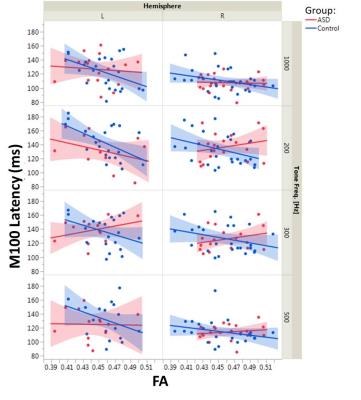
DTI tractography of the brainstem auditory pathway was successfully performed through the lateral lemniscus and brachium of inferior colliculus (Figure 1). Maturation of FA (p<0.005, increases with age) and RD (p<0.05, decreases with age) was observed. No significant effect of group, hemisphere or interaction terms was observed for any DTI measure. Similarly, M100 was observed to decrease with age (p<0.0001) and M100 latencies were later in the left than the right hemisphere (p<0.002). No significant effect on M100 of group or the interaction terms was observed. For the multimodal analysis, increased FA was predictive of faster conduction (lower M100 latency, p<0.01). Increased MD and RD were correlated with increased M100 latency (p<0.01, each). The interaction terms of FA, MD, and RD with group were significant (p<0.05 for FA and MD, p=0.05 for RD), indicating an altered structure-function relationship in ASD. Examined separately by group, DTI measures were correlated with M100 latency in the typically developing controls but not the ASD group. Plots of M100 latency versus FA for each hemisphere, tone frequency, and group are shown in Figure 2.Discussion/Conclusions
This multimodal study examined the structure of early auditory sensory pathways as well as associations between these pathways and the M100 latency. The lack of microstructural lateralization of the brainstem auditory pathway indicated a lack of functional lateralization at this early stage of the auditory system. Degree of brainstem auditory pathway myelination modulated the speed of auditory encoding in controls. Although both DTI and MEG measures were observed to undergo maturation, a dissociation of the relationship between structure and function was observed in ASD. These results contribute to our understanding of ASD as a disorder that impacts structure-functions relationships throughout the auditory system. Although DTI is capable of examining microstructure along a large portion of the auditory system’s course within the brainstem and cerebrum, other factors such as grey matter synapses and cortical organization also contribute to the efficiency of the auditory system.Acknowledgements
This work was supported by K01MH096091, R01DC008871-07, and the CHOP/UPenn IDDRC grant U54 HD086984.References
1. Prigge, M.D., et al., Longitudinal Heschl's Gyrus Growth During Childhood and Adolescence in Typical Development and Autism. Autism Research, 2013. 6(2): p. 78-90.
2. Lange, N., et al., Atypical diffusion tensor hemispheric asymmetry in autism. Autism Research, 2010. 3(6): p. 350-358.
3. Roberts, T.P.L., et al., MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Research, 2010. 3(1): p. 8-18.
4. Berman, J.I., et al., Multimodal Diffusion-MRI and MEG Assessment of Auditory and Language System Development in Autism Spectrum Disorder. Frontiers in Neuroanatomy, 2016. 10: p. 30.
5. Roberts, T.P.L., et al., Maturational differences in thalamocortical white matter microstructure and auditory evoked response latencies in autism spectrum disorders. Brain Res, 2013.
6. Miron, O., et al., Prolonged auditory brainstem responses in infants with autism. Autism Research, 2015.
Figures