2249
Cerebral perfusion in Schizophrenia: an Arterial Spin Labeling study1Physics Department, University of Sao Paulo, Ribeirao Preto, Brazil, 2Department of Neuroscience and Behavioral Sciences, University of Sao Paulo, 3Department of Medical Clinic, University of Sao Paulo
Synopsis
Schizophrenia is a mental disorder with structural and functional alterations that are not completely comprehended. We used a pseudo-continuous ASL protocol to investigate changes in resting CBF in schizophrenic patients. In addition, quantitative T1 and Gray Matter Volume (GMV) were assessed. Decreased CBF, GMV, and T1 values were observed in schizophrenic patients compared with healthy controls in several brain regions consistent with patients’ symptoms, such as deficits in planning solving and organizing thoughts. Therefore, motor, sensorial and cognitive impairments observed in schizophrenia may be related to CBF deficits and structural alterations in localized brain regions.
Purpose
Schizophrenia is a mental disorder that affects several brain structures and functions1, but its etiology is not completely comprehended. Therefore, the purpose of the present study is to investigate brain regions with compromised Cerebral Blood Flow (CBF) in schizophrenic patients using pseudo-continuous Arterial Spin Labeling (pCASL), and its correlation with structural brain alterations and patients’ symptoms.Methods
Twenty schizophrenic patients (19–45 years old, 16 men), recruited at the Clinical Hospital of Ribeirao Preto, and twenty healthy controls (19–46 years old, 12 men, no history of psychoses episode) participated in the study, and gave written consent after being informed about the experimental procedures, previously approved by a committee of ethics in research. Patients with schizophrenic diagnoses (PANSS score ≥ 4 per item) and in treatment for the presence of positive or negative symptoms were evaluated by a trained psychiatrist. Imaging was performed on a 3T Philips System (Achieva, The Netherlands) using a 32-channel head coil for reception. pCASL images were obtained using a 2D single-shot EPI sequence with the following parameters: TR/TE = 4000/14ms, FA = 90°, FOV = 240x240mm2, matrix = 160x160, 20 5-mm slices, label duration/post-labeling delay = 1650/1525ms, 35 control/label pairs. Anatomical 3D-T1 images were acquired with the following parameters: TR/TE= 7/3.2ms, FA = 8°, matrix = 240x240, FOV = 240x240mm2, 160 1-mm slices. Quantitative T1 maps were obtained using a Look-Locker sequence with 12 different inversion times and the following parameters: TR/TE = 4600/2.3ms, FA = 8°, matrix = 64x64, FOV = 240x240 mm2, 20 5-mm slices. Data processing was performed using Statistical Parametric Mapping (SPM12) and a toolbox for ASL (ASLtbx)2. For Gray Matter Volume (GMV) quantification, SPM12-DARTEL registration method3 was used. Two-way ANOVA, corrected for multiple comparisons, was used to compare regional CBF values between groups. A voxel-wise analysis was performed to compare quantitative T1 maps.Results
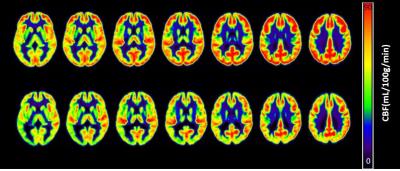
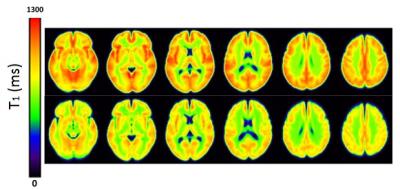
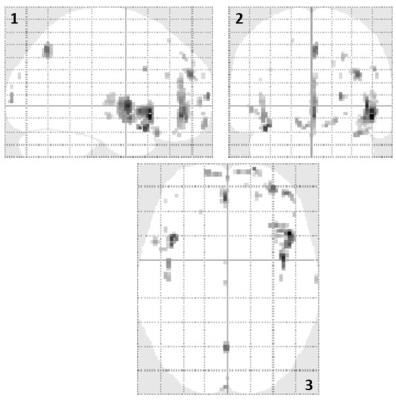
Mean CBF value for gray matter was significantly reduced in schizophrenic patients (60 ± 9 mL/100g/min) compared to healthy controls (68 ± 11 mL/100g/min, p = 0.018) (Figure 1). The same difference was observed in several brain regions in both hemispheres: superior, middle and inferior frontal gyri; supplementary motor and superior medial frontal areas; superior and inferior parietal gyri; angular gyrus; superior, middle e inferior occipital gyri; anterior, middle and posterior cingulate gyri; cuneus and precuneus. Left precentral and postcentral gyri, and left middle temporal gyrus also showed CBF deficits in patients. On the other hand, CBF values tended to be increased in patients in four regions: amygdala, putamen, pallidum and thalamus. Moreover, decreased gray matter T1 values were also observed for patients compared with healthy controls (Figure 2). A voxel-wise analysis showed regional T1 differences, mainly in frontal areas and cingulate gyri (Figure 3), where CBF was also reduced. Besides, we observed decreased GMV in patients (0.67 ± 0.07 L) compared with healthy controls (0.72 ± 0.06 L, p < 0.011). Finally, correlating imaging results with the duration of the disease and PANSS scores, we observed that CBF in a small area of the right frontal gyrus (rolandic operculum) is negatively correlated with the duration of the disease, and CBF in the right hippocampus is positively correlated with PANSS negative scores.Discussion
We identified decreased CBF in several brain regions consistent with prior findings in schizophrenia using PET, SPECT and ASL1,4, and these regions are involved with different tasks reported to be compromised in schizophrenic patients. For example, the frontal lobe is involved with critical problem solving, comprehension, and other high-level reasoning as planning actions and organizing thoughts. More specifically, CBF in the rolandic operculum, involved with motor imagery and verbalization5,6, reduces with the duration of the disease. Other regions showing reduced CBF also have direct relationship with the patients’ symptoms, such as motor disturbances; deficits in visual perception, spatial orientation and language processing; and emotion. On the other hand, increased CBF in regions of the limbic system may be related to drugs used for the patients’ treatment. Moreover, since T1 relaxation is sensitive to changes in myelin, decreased T1 values may be related to changes in myelin7. Previous studies in schizophrenic patients showed a relationship between myelination and the critical period for the symptoms onset in schizophrenia8; however, quantitative T1 is not specific to myelination, and other MRI techniques should be used.Conclusion
Motor, sensorial and cognitive impairments observed in schizophrenic patients may be somehow related to CBF deficits and structural alterations in localized brain regions. Then, structural and functional connectivity will also be investigated to better understand these results.Acknowledgements
No acknowledgement found.References
1. Zhu J, Zhuo C, Qin W, et al. Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J Psychiatr Res. 2015;63:28-35.
2. Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26(2):261-269.
3. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95-113.
4. Ota M, Ishikawa M, Sato N, et al. Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophr Res. 2014;154(1-3):113-118.
5. Szameitat AJ, McNamara A, Shen S, Sterr A. Neural activation and functional connectivity during motor imagery of bimanual everyday actions. PLoS One. 2012;7(6):e38506.
6. Zarnhofer S, Braunstein V, Ebner F, et al. The Influence of verbalization on the pattern of cortical activation during mental arithmetic. Behav Brain Funct. 2012;8:13.
7. Lutti A, Dick F, Sereno MI, Weiskopf N. Using high-resolution quantitative mapping of R1 as an index of cortical myelination. Neuroimage. 2014;93 Pt 2:176-188.
8. Chambers JS, Perrone-Bizzozero NI. Altered myelination of the hippocampal formation in subjects with schizophrenia and bipolar disorder. Neurochem Res. 2004;29(12):2293-2302.
Figures