2244
Microstructure Alterations of Earthquake Survivors: A Longitudinal MR Diffusion Study1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu, People's Republic of China
Synopsis
To reveal how traumatic events affect the integrity of brain microstructure in trauma-exposed non-PTSD people, we performed a longitudinal tract-based spatial statistics (TBSS) analysis in earthquake survivors using diffusion tensor imaging (DTI) data collected 25 days (TimePoint 1) and 2 years (TimePoint 2) after the Wenchuan earthquake. Our results showed that fractional anisotropy (FA) in several brain regions at TimePoint 2 were significantly increased compared with those at TimePoint 1. The increased FA in these regions may serve as the underlying neural substrates as brain recovered from the trauma.
Background
The consequences of exposure to extreme traumatic events (i.e., natural disaster, accident, physical or sex abuse, combat), have influenced more and more individuals around the world1,2. Among trauma-exposed individuals, however, only a minority of them will develop full-blown posttraumatic stress disorder (PTSD) which is defined by sustained symptoms for more than one month following exposure3,4. Many studies have focused on brain changes in PTSD. Nevertheless, fewer studies have explored structural and functional changes in trauma-exposed non-PTSD people, although there are evidences that brain can be affected by psychological stress 1,5-9. So far, it is not clear how the white matter varies in trauma-exposed non-PTSD people. Therefore, in this investigation, we performed a longitudinal tract-based spatial statistics (TBSS) analysis in earthquake survivors using DTI data collected 25 days (TimePoint 1) and 2 years (TimePoint 2) after the Wenchuan earthquake.Methods
A total of 22 survivors of the Wenchuan earthquake (Mercalli intensity scale: 8.0) recruited from the most affected geographic regions were enrolled in our study. The participants underwent magnetic resonance imaging (MRI) scanning twice: within 25 days (TimePoint 1) of the earthquake and two years later (TimePoint 2). All DTI datasets were obtained with GE 3.0T MR scanner. Voxel-wise statistical analysis of DTI data was carried out usingTBSS within FSL (http://www.fmrib.ox.ac.uk/fsl). We examined the diffusional characteristics of the significant clusters using the diffusion parameters including fractional anisotropy (FA), axial diffusivity (AD) and radial diffusivity (RD). Finally, deterministic DTI tractography was performed in MedInria software.Results
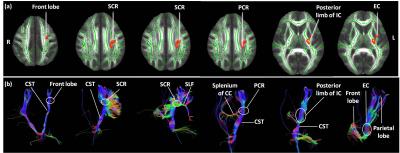
Voxel-wise statistics (p < 0.01 and a cluster size > 10 voxels) found that FA in left posterior limb of internal capsule, left posterior corona radiate, left superior corona radiate, left external capsule and frontal lobe(Fig.1) at TimePoint 2 were significantly increased compared those at TimePoint 1. There was no significant difference in AD, while RD in left posterior limb of internal capsule, left superior longitudinal fasciculus (SLF) and frontal lobe (Fig.1) at TimePoint 2 were significantly decreased compared those at TimePoint 1. The tractography analysis indicated that the clusters of increased FA were located at the corticospinal tract, SLF, splenium of the corpus callosum and the crossing fibers from the frontal lobe and parietal lobe (Fig.2).Discussion
Using voxel based approach in trauma-exposed non-PTSD people who experienced similar stress, the present investigation reveals how traumatic events affect the integrity of brain microstructure. Combined with our previous study7, the increased FA may represent the recovering of brain microstructure. Furthermore, our study provides evidence that alterations in brain white matter microstructure are expressed in corticospinal tract, SLF and external capsule. The corticospinal tracts mainly contain motor axons projecting from primary motor cortex. The SLF is a primary association tract connecting the frontal, parietal, temporal cortex and limbic circuits, with a particular bias toward fibers originating or terminating in the dorsal lateral prefrontal cortex10, 11. Meanwhile, these regions associating with trauma-related mental illness have been well established3,10-16. Furthermore, the tractography analysis indicated that the clusters of increased FA in the corticospinal tract, SLF and external capsule, received the most robust projections from the temporal lobe, splenium of the corpus callosum, frontal lobe and parietal lobe (especially sensorimotor), which have been revealed to associate with stress1,7,17,18. Thus, our study provides more evidence to confirm the mediating role of those regions in emotion regulation.Conclusion
The present study using TBSS provides evidence of the longitudinal changes in brain white matter microstructure in trauma-exposed non-PTSD people, and explores the underlying neural substrates as brain recovered from the trauma.Acknowledgements
This work was supported by the National Natural Science Foundation (Grant No. 81401477, 81030027, 81227002 and 81220108013), National Key Technologies R&D Program (Program No. 2012BAI01B03) and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No. IRT1272) of China.References
1. Du MY, Liao W, Lui S, et al. Altered functional connectivity in the brain default-mode network of earthquake survivors persists after 2 years despite recovery from anxiety symptoms. Soc Cogn Affect Neurosci. 2015;10(11):1497-1505.
2. Satcher D, Friel S, Bell R. Natural and manmade disasters and mental health. Jama. 2007;298(21):2540-2542.
3. Ding AY, Li Q, Zhou IY, et al. MR diffusion tensor imaging detects rapid microstructural changes in amygdala and hippocampus following fear conditioning in mice. PLoS One. 2013;8(1):e51704.
4. Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56(1):19-32.
5. Holzel BK, Carmody J, Evans KC, et al. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2010;5(1):11-17.
6. Soares JM, Sampaio A, Ferreira LM, et al. Stress-induced changes in human decision-making are reversible. Transl Psychiatry. 2012;2:e131.
7. Chen L, Lui S, Wu QZ, et al. Impact of acute stress on human brain microstructure: An MR diffusion study of earthquake survivors. Hum Brain Mapp. 2013;34(2):367-373.
8. Paul R, Henry L, Grieve SM, et al. The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatr Dis Treat. 2008;4(1):193-201. 9. Lu S, Wei Z, Gao W, et al. White matter integrity alterations in young healthy adults reporting childhood trauma: A diffusion tensor imaging study. Aust N Z J Psychiatry. 2013;47(12):1183-1190.
10. Korgaonkar MS, Grieve SM, Koslow SH, Gabrieli JD, Gordon E, Williams LM. Loss of white matter integrity in major depressive disorder: evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum Brain Mapp. 2011;32(12):2161-2171.
11. Zuo N, Fang J, Lv X, et al. White matter abnormalities in major depression: a tract-based spatial statistics and rumination study. PLoS One. 2012;7(5):e37561.
12. Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201(2):239-243.
13. Boccia M, D'Amico S, Bianchini F, Marano A, Giannini AM, Piccardi L. Different neural modifications underpin PTSD after different traumatic events: an fMRI meta-analytic study. Brain Imaging Behav. 2015.
14. Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2(1):9.
15. Li L, Lei D, Li L, et al. White Matter Abnormalities in Post-traumatic Stress Disorder Following a Specific Traumatic Event. EBioMedicine. 2016;4:176-183.
16. Daniels JK, Lamke JP, Gaebler M, Walter H, Scheel M. White matter integrity and its relationship to PTSD and childhood trauma--a systematic review and meta-analysis. Depress Anxiety. 2013;30(3):207-216.
17. Lui S, Huang X, Chen L, et al. High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proc Natl Acad Sci U S A. 2009;106(36):15412-15417.
18. Coplan JD, Kolavennu V, Abdallah CG, et al. Patterns of anterior versus posterior white matter fractional anistotropy concordance in adult nonhuman primates: Effects of early life stress. J Affect Disord. 2016;192:167-175.
Figures