2241
Compulsivity as a Transdiagnostic Trait in Humans and Animal Models1Centre for Neuroimaging Sciences, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom, 2Department of Cognitive Neurscience, Radboud University Medical Center, Nijmegen, Netherlands, 3Center for Image Sciences, University Medical Center Utrecht, Utrecht, Netherlands, 4Department of Cognitive Neuroscience, Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, Netherlands, 5Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht, Netherlands, 6Department of Child and Adolescent Psychiatry and Psychotherapy, Central Institute of Mental Health Mannheim, Mannheim, Germany, 7NatBrainLab, Department of Forensics and Neurodevelopmental Sciences, Sackler Institute of Translational Neuroimaging, King's College London, London, United Kingdom
Synopsis
Obsessive-compulsive disorder (OCD) and diabetes mellitus type 2 (DM2) show compulsive behaviour1 and share genetic vulnerability2. Using Diffusion Tensor Imaging as a translational approach, we investigated differences in corpus callosum (CC) body white matter microstructure in a paediatric human OCD cohort and juvenile animal models for OCD and DM2. In all three groups, fractional anisotropy increased in the CC body compared to controls, which correlated with increasing compulsive behaviour. This was coupled with a decrease in CC mean diffusivity in the animal models. Our results underline the importance of compulsive behaviour as a possible trans-diagnostic trait across OCD and DM2.
Purpose
Obsessive-compulsive disorder (OCD) is a psychiatric neurodevelopmental disorder characterised by obsessive and compulsive behaviour with a lifetime prevalence of 2-4%3. Compulsive behaviour is also frequent in patients with diabetes mellitus type 2 (DM2)1, a somatic disorder it shares genetic vulnerability with2. While animal models for both disorders have been developed, direct translation of findings from animal models to humans has been missing. We chose in vivo Diffusion Tensor Imaging (DTI) as a translational approach for both a human paediatric OCD cohort and animal models of OCD and DM2. We used a statistically-based analysis approach to identify white matter regions with increased fractional anisotropy (FA) values and which increased with compulsive behaviour scores in the human cohort allowing us to draw white matter regions of interest in the juvenile animal models.Methods
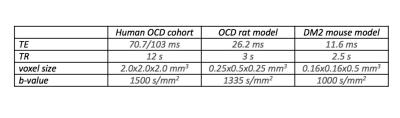
Human cohort: Thirty-seven gender-matched participants aged 9-14 years (Patients diagnosed with OCD4: n=11, Controls: n=26) were included (Table 1) and compulsive behaviour was measured through the Repetitive Behaviour Scale (RBS)5. OCD rat model: We used quinpirole treatment to model juvenile OCD in rats6. Thirty-seven, five week-old Sprague Dawley rats were subcutaneously injected with the D2/3-dopamine receptor agonist quinpirole hydrochloride (0.5 mg/kg; OCD: n=19), or saline (Controls: n=18), twice a week for 6 weeks. Directly after quinpirole/saline injections, animals were placed in an open field arena and compulsive behaviour was assessed by the number of visits to the most frequently visited zone. MR data was acquired on a 9.4T scanner 1-3 days before the first (t0), and 90 minutes after the 10th (t1) and 12th (t2) injections of quinpirole/saline (Table 1). TALLYHO mouse model: We chose the polygenic TALLYHO/JngJ (TH) type 2 diabetes mellitus mouse model with SWR/J mice as controls7. Eighteen male mice (TH: n=9, Controls: n=9) were allowed to explore a Y-maze freely, where a reduction in spontaneous alternation of distal arm maze entries reflected increased compulsive behaviour. Data was acquired on a 11.7T scanner (Table1). DTI Analysis For the human data, FSL’s randomise was used on whole brain white matter to perform analyses of correlation with RBS and identify regions where the OCD patient group displayed higher FA values than the control group. All analyses included age, gender and scanning site as covariates and were family-wise error corrected for multiple comparisons. The main white matter region displaying higher FA values in the humans was replicated in both animal models as region of interest using ImageJ to define and extract values. Two subsequent scans were averaged for analysis with the CBW J13 MR-histology rat atlas8 and Allen Brain Mouse Atlas9 respectively for orientation. Pre-processing for both human and animal data was done using ExploreDTI10, generating FA and Mean Diffusivity (MD) maps. Independent t-tests determined changes in FA/MD, while Spearman’s rho nonparametric correlation analyses related FA/MD changes to behavioural changes. All statistical analyses were performed using SPSS and results were Bonferroni corrected for multiple comparisons when applicable.Results
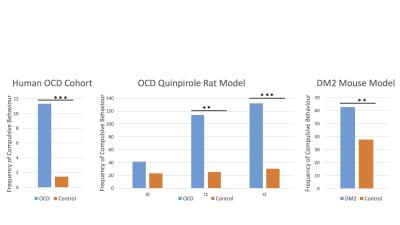
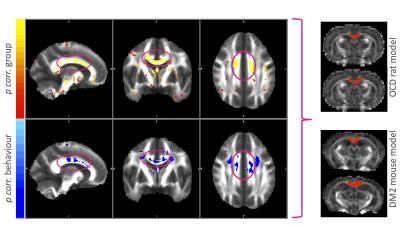
All three disease modelling cohorts showed increased compulsive behaviour (human OCD: p<.001, rat OCD at T2: p=.001; rat OCD at T3: p<.001; DM2 mice: p=.003; Figure 1). In the human OCD group, the body of the corpus callosum (CC) displayed increased FA values on a group level, which were positively correlated with compulsive behaviour (Figure 2), leading to this structure being used as a region of interest for the animal models. Both animal models displayed an increase in FA (OCD: p=.002; DM2: p=.000) coupled with a decrease in MD (OCD: p<.001; DM2: p=.024) in the body of the corpus callosum. CC body FA values were positively correlated with compulsive behaviour (OCD: r=.172, p=.202; DM2: r=.481, p=.043) and negatively correlated with MD values (OCD: r=-.377, p=.004; DM2: r=-.473, p=.047).Discussion and Conclusion
FA increases and MD decreases in the CC body were associated with compulsive behaviour across species for both somatic and psychiatric animal models. Our results underline the importance of compulsive behaviour as a possible trans-diagnostic trait independent of the nature of the disorders. Shared genetic vulnerability across both disorders2 suggests possible abnormal juvenile brain development of CC body white matter microstructure in both disorders and possibly other disorders displaying increased compulsive behaviour. Although compulsive behaviour patterns might be mediated by different neural pathways, the consistent FA increase/MD decrease pattern we found suggests the CC body as a converging brain region. To define the exact nature of the white matter abnormalities found, we plan to investigate additional regions of interest, include other disorder models with increased compulsive behaviour as well as histological validation in future studies.Acknowledgements
No acknowledgement found.References
1. Levitt Katz, L. E. et al. Neuropsychiatric disorders at the presentation of type 2 diabetes mellitus in children. Pediatric Diabetes 6, 84–89 (2005).
2. van de Vondervoort, I., Poelmans, G. & Aschrafi, A. An integrated molecular landscape implicates the regulation of dendritic spine formation through insulin-related signalling in obsessive–compulsive disorder. J Psychiatry Neurosci 41, 280-285 (2016).
3. Stein, D. J. Obsessive-compulsive disorder. The Lancet 360, 397–405 (2002).
4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed., 2013).
5. Lam, K. S. L. & Aman, M. G. The Repetitive Behavior Scale-Revised: Independent Validation in Individuals with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders 37, 855–866 (2007).
6. Szechtman, H., Sulis, W. & Eilam, D. Quinpirole induces compulsive checking behavior in rats: A potential animal model of obsessive-compulsive disorder (OCD). Behavioral Neuroscience 112, 1475–1485 (1998).
7. Kim, J. H. & Saxton, A. M. The TALLYHO mouse as a model of human type 2 diabetes. Methods Mol. Biol. 933, 75–87 (2012).
8. Calabrese, E., Badea, A., Watson, C. & Johnson, G. A. A quantitative magnetic resonance histology atlas of postnatal rat brain development with regional estimates of growth and variability. NeuroImage 71, 196–206 (2013).
9. Dong, H. W. The Allen reference atlas: A digital color brain atlas of the C57Bl/6J male mouse. (John Wiley & Sons Inc, 2008).
10. Leemans, A., Jeurissen, B. & Sijbers, J. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc. Intl. Soc. Mag. Reson. Med. 17 (2009).
Figures