2239
Muscarinic receptor agonism prevents the functional connectivity aberrancies produced by the psychotogenic drug phencyclidine (PCP)1Functional Neuroimaging Laboratory, Center for Neuroscience and Cognitive Systems @ Unitn, Istituto Italiano di Tecnologia, Rovereto, Italy, 2CIMeC, Center for Mind/Brain Sciences, University of Trento, Rovereto, Italy, 3Psychological and Brain Sciences, Indiana University, Bloomington, IN, United States, 4Radiology and Imaging Sciences, Indiana University, Indianapolis, IN, United States
Synopsis
NMDA receptor antagonists like ketamine or phencyclidine (PCP) induce robust schizophrenia-like symptoms in rodents via glutamatergic disinhibition of cortico-limbo-thalamic substrates. We show that acute administration of PCP in the mouse elicits aberrant fronto-hippocampal and thalamo-cortical functional connectivity, an effect that can be prevented by pharmacological activation of M1/M4 muscarinic receptors. These changes highlight a previously unreported permissive contribution of muscarinic receptors on the aberrant connectional signatures produced by NMDAr antagonism which bear relevance for human connectivity mapping in hyperglutamatergic states and schizophrenia.
Background
Functional magnetic resonance imaging (fMRI) techniques offer the potential to detect changes in brain function elicited by a pharmacological intervention and, as such, improve translatability of pharmacological mechanism from preclinical models to clinical outcome. Xanomeline, a M1/M4 preferring muscarinic receptor agonist 1–4 has shown putative pro-cognitive effects in schizophrenic patients 5–9. To mechanistically probe the pharmacological action of this drug, here we mapped its ability to modulate or prevent the aberrant functional cascade produced by sub-anesthetic dose of the NMDA receptor antagonist PCP, a drug pharmacologically related to ketamine that produces robust schizophrenia-like syndromes and stereotypical pharmacological and resting-state fMRI (phMRI and rsfMRI, respectively) responses in rodents 10–13.Methods
All experiments were carried out in accordance with Italian regulations governing animal welfare and protection. MRI experiments were performed on male C57Bl6/J mice (n=40). Animal preparation: The procedure employed for rsfMRI has been recently described. Briefly, mice were anaesthetized with isoflurane (5%), intubated, and artificially ventilated. rsfMRI timeseries were acquired using controlled halothane anesthesia (0.7%). rsfMRI: All experiments were performed using a 7.0 Tesla MRI scanner using a single-shot EPI sequence with TR/TE 1200/15 ms, matrix 100 × 87, field of view 2.3 × 2 cm2, 16 coronal slices, slice thickness 0.75 mm and NT=1500. rsfMRI data analysis: rsfMRI time series were pre-processed (registered, motion regressed band-pass filtered; 0.1-0.01 Hz and smoothed) as recently described (1). Mice were randomly assigned to one of the following treatment groups:
- Xanomeline – PCP (n=10),
- Xanomeline – vehicle (n=8),
- Vehicle – vehicle (n=9),
- Vehicle – PCP (n=10).
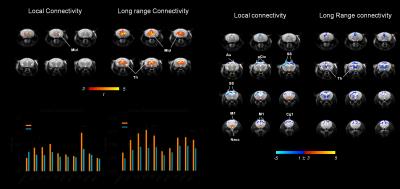
The phMRI response was mapped and quantified as previously described 14. To obtain an unbiased identification of the brain regions exhibiting differences given by the treatment (xanomeline or PCP) in functional connectivity, we calculated long range connectivity (GLBC, global connectivity minus local connectivity) and local brain connectivity (LBC) maps for all subjects 15,16. Networks were mapped using seed-based approach as previously described 17.
Results
Pharmacological fMRI
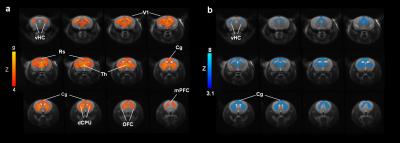
Acute administration of PCP induced robust activation of a previously described set of cortico–limbo–thalamic regions (Fig. 1; 12) encompassing the cingulate, medial prefrontal, orbito-frontal and retrosplenial cortices, with extension into the primary visual cortex. Pretreatment with xanomeline robustly attenuated the BOLD response to PCP in several brain regions recapitulating large portions of the phMRI activation pattern observed with the NMDA antagonist (Fig. 1).
Resting-state fMRI
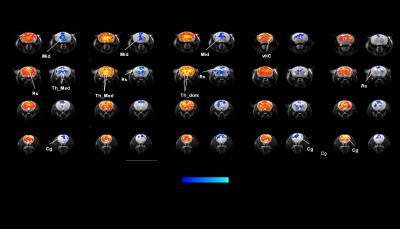
Administration of PCP produced prominent increases in thalamic and midbrain long-range connectivity, recapitulating similar findings in humans with ketamine. Small foci of increased local connectivity were observed in midbrain regions (Fig. 2). The administration of PCP also results in increased rsfMRI connectivity in specific network systems, namely the mouse default mode network (DMN) and thalamo-cortical networks. Importantly, xanomeline pre-treated subjects exhibited reduced thalamic global connectivity (Fig. 3), thus effectively preventing PCP-induced thalamic hyper-connectivity. The drug also inhibited PCP-induced connectivity increase in several brain networks (Fig. 3), with prominent effects in thalamic systems and regions, antero-posterior regions of the mouse default brain network, including fronto-hippocampal areas.
Discussion
Both the phMRI response and rsfMRI network activity mapped upon PCP administration are consistent with the result of previous rodent and human mapping of NMDA receptor antagonism, most of which have been carried out with the PCP-analogue ketamine 18–22. The ability of xanomeline to prevent the functional effects of PCP is consistent with a putative anti-psychotic action of this compound, and suggest that normalization of schizophrenia-like behavior exerted by this drug may entail the regularization of aberrant network activity. More broadly, the phMRI and rsfMRI data in the present study provide strong evidence for a major modulatory role of muscarinic M1/M4 receptors in the brain’s functional connectivity, and suggest that this receptor system exert a permissive contribution on the aberrant glutamatergic cascade produced by NMDAr antagonism. From a technical standpoint, our findings support the combined use of phMRI and rsfMRI as pharmaco-dynamic biomarkers for the determination of central biological activity in human clinical studies, and mechanistic dissection of centrally-active compounds.Acknowledgements
No acknowledgement found.References
1. Shannon, H. E. et al. Xanomeline, an M1/M4 preferring muscarinic cholinergic receptor agonist, produces antipsychotic-like activity in rats and mice. Schizophr. Res. 42, 249–259 (2000).
2. Mirza, N. R., Peters, D. & Sparks, R. G. Xanomeline and the Antipsychotic Potential of Muscarinic Receptor Subtype Selective Agonists. 9, 159–186 (2003).
3. Andersen, M. B. et al. The muscarinic M1/M4 receptor agonist xanomeline exhibits antipsychotic-like activity in Cebus apella monkeys. Neuropsychopharmacology 28, 1168–1175 (2003).
4. HE, S. et al. Xanomeline, an M(1)//M(4) preferring muscarinic cholinergic receptor agonist, produces antipsychotic-like activity in rats and mice. Schizophr Res 42, 249–259 (2000).
5. Cui, Y. H. et al. A novel derivative of xanomeline improved memory function in aged mice. Neurosci. Bull. 24, 251–257 (2008).
6. Si, W. et al. A novel derivative of xanomeline improves fear cognition in aged mice. Neuroscience Letters 473, (2010).
7. Wang, D. et al. Attenuation of neurodegenerative phenotypes in Alzheimer-like presenilin 1/presenilin 2 conditional double knockout mice by EUK1001, a promising derivative of xanomeline. Biochemical and Biophysical Research Communications 410, (2011).
8. NC, B. et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol 54, 465–473 (1997).
9. Avery, E. E., Baker, L. D. & Asthana, S. Potential Role of Muscarinic Agonists in Alzheimer’s Disease. Drugs Aging 11, 450–459 (1997).
10. Farber, N. B. The NMDA receptor hypofunction model of psychosis. Ann N Y Acad Sci 1003, 119–130 (2003).
11. Greene, R. Circuit analysis of NMDAR hypofunction in the hippocampus, in vitro, and psychosis of schizophrenia. Hippocampus 11, 569–577 (2001).
12. Gozzi, A. et al. Differential Effects of Antipsychotic and Glutamatergic Agents on the phMRI Response to Phencyclidine. Neuropsychopharmacology 33, 1690–1703 (2008).
13. Gass, N. et al. Sub-anesthetic ketamine modulates intrinsic BOLD connectivity within the hippocampal-prefrontal circuit in the rat. Neuropsychopharmacology 39, 895–906 (2013).
14. Errico, F. et al. A role for D-aspartate oxidase in schizophrenia and in schizophrenia-related symptoms induced by phencyclidine in mice. Transl Psychiatry 5, e512 (2015).
15. Liska, A., Galbusera, A., Schwarz, A. J. & Gozzi, A. Functional connectivity hubs of the mouse brain. Neuroimage 115, 281–291 (2015).
16. Cole, M. W., Pathak, S. & Schneider, W. Identifying the brain’s most globally connected regions. Neuroimage 49, 3132–3148 (2010).
17. Sforazzini, F., Schwarz, A. J., Galbusera, A., Bifone, A. & Gozzi, A. Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. Neuroimage 87, 403–415 (2014).
18. De Simoni, S. et al. Test–retest reliability of the BOLD pharmacological MRI response to ketamine in healthy volunteers. Neuroimage 64, 75–90 (2013).
19. Doyle, O. M. et al. Quantifying the Attenuation of the Ketamine Pharmacological Magnetic Resonance Imaging Response in Humans: A Validation Using Antipsychotic and Glutamatergic Agents. J. Pharmacol. Exp. Ther. 345, 151–160 (2013).
20. Deakin, J. F. W. et al. Glutamate and the Neural Basis of the Subjective Effects of Ketamine: A Pharmaco-Magnetic Resonance Imaging Study. Arch. Gen. Psychiatry 65, 154–164 (2008).
21. Schwarz, A. J., Gozzi, A., Reese, T., Heidbreder, C. A. & Bifone, A. Pharmacological modulation of functional connectivity: the correlation structure underlying the phMRI response to d-amphetamine modified by selective dopamine D3receptor antagonist SB277011A. Magn. Reson. Imaging 25, 811–820 (2007).
22. Becker, R. et al. Species-conserved reconfigurations of brain network topology induced by ketamine. Transl. Psychiatry 6, e786 (2016).
Figures