2235
Pharmacological Inhibition of ERK Pathway Rescues Morpho-Anatomical Aberrancies Associated with 16p11.2 Chromosomal Deletion1Functional Neuroimaging Laboratory, Center for Neuroscience and Cognitive Systems, Istituto Italiano di Tecnologia, Rovereto, Italy, 2CIMeC, Center for Mind/Brain Sciences, University of Trento, Rovereto, Italy, 3Department of Neurosciences, Case Western Reserve University, Cleveland, OH, 4School of Biosciences, Cardiff University, Cardiff, United Kingdom
Synopsis
16p11.2 microdeletion is the most common copy number variation in autism. Recent studies revealed that mice harboring this microdeletion exhibit a paradoxical elevation of ERK activity, macroscale gray matter abnormalities and autistic-like behavioral deficits. By using high-resolution morpho-anatomical MRI, we show that prenatal treatment with an ERK pathway inhibitor rescues hippocampal and septal anatomical deficits in 16p11.2del mutants. The effect was associated with amelioration of anxiety behaviors. These results provide the first example of the rescue of developmental gray matter abnormalities in this mouse model, and support the translational use of structural MRI to assess putative therapeutic effects in autism.
BACKGROUND
Copy number variations (CNV) are associated with approximately 10-20% of Autism Spectrum Disorder (ASD). 16p11.2 is the most common CNV in ASD, with individuals heterozygous for the 16p11.2 deletion exhibiting a range of clinical symptoms including cognitive impairments, hyperactivity, anxiety and epilepsy1. The human 16p11.2 locus contains 27 genes, which includes the MAPK3 gene (encoding ERK1) and the Major Vault Protein gene (MVP), both of which converge onto the ERK/MAP kinase pathway2. The ERKs play critical roles brain development and synaptic plasticity3 in response to a broad range of stimuli4. It was previously reported that a murine model of the 16p11.2 human microdeletion (16p11.2del) exhibits a reduction in brain size and perturbations in cortical cytoarchitecture, which are largely due to ERK-mediated regulation of neural progenitor proliferation5 . Importantly, 16p11.2del mice exhibit a paradoxical increase in ERK signaling5 coincident with aberrant cortical neurogenesis and macroscale volumetric rearrangements6 ultimately resulting in behavioral deficits analogous to the 16p11.2 microdeletion carriers7. Therefore, we postulated that treatment with a brain permeant ERK pathway inhibitor8 may correct brain volumetric alterations associated with the 16p11.2 deletion. To probe whether such anatomical defects could be reversed by drug treatment and identify a putative translational endpoint for these measurements, we used high-resolution morphoanatomical MRI to map gray matter volume in 16p11.2del mice upon prenatal treatment with an ERK inhibitor. In the same mice we also probed network structure of gray matter using structural covariance mapping9, an approach that we term structural covariance MRI (scMRI).METHODS
All experiments were carried out in accordance with Italian regulations governing animal welfare and protection. High-resolution morpho-anatomical T2-weighted MR imaging of ex-vivo mouse brains was performed in paraformaldehyde fixed specimens [10]. MRI image were acquired at 7 Tesla using a FLASH 3D sequence with TR = 17 ms, TE = 10 ms, α = 30°, matrix size of 260 × 180 × 180 and voxel size of 0.07 mm (isotropic)10. Inter-group morpho-anatomical differences in local volumes were mapped with TBM using ANTs, and volumetric measurements were independently computed via automated anatomical labelling as we recently described11 using two neuroanatomically parcellated reference MRI atlases12,13. To investigate changes in the trophic dynamics between brain regions due to 16p11.2del CNV we calculated and modulated gray matter tissue probability maps, to carry out seed based structural scMRI mapping14, using unilateral mean gray matter volumes of representative regions as regressors (t > 3, pc = 0.01). We also tested the 16p11.2del and WT control mice with elevated-plus maze and open field to assess anxiety.RESULTS AND DISCUSSION
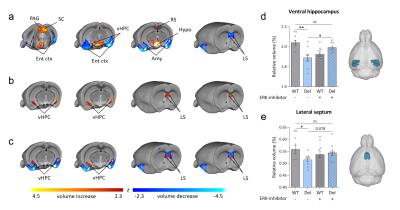
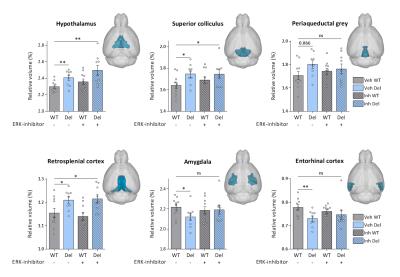
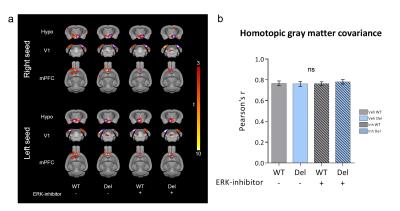
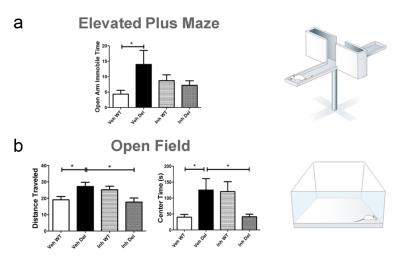
Consistent with previous reports6, 16p11.2del mice showed increases in the relative volume of the hypothalamus, superior colliculus and periaqueductal grey when compared to WT mice (Fig. 1-2). Voxelwise TBM mapping also revealed foci of decreased volume in ventral hippocampal, amygdalar, entorhinal and lateral septal areas in 16p11.2del mice when compared to WT controls (Fig. 1). Importantly, treatment with the ERK inhibitor rescued ventral hippocampal and lateral septal volume in 16p11.2del mice when compared to vehicle treated controls (Fig. 1). Interestingly, the ventral hippocampus is a region where pERK is highly expressed in 16p11.2del mice during mid-neurogenesis and a key substrate for anxiety-related behavior14. This led us to probe anxiety traits in control and treated 16p11.2del mice. Control 16p11.2del mice showed pronounced freezing behavior in both elevated plus maze and in the open field test. In keeping with the imaging results, this hippocampal based behavior was ameliorated by ERK inhibitor treatment in both settings (Fig. 3). Finally, we mapped structural covariance (scMRI) networks with seed region analysis to probe alterations in the architecture of gray matter correlations between brain regions. Our data revealed that 16p11.2del mice exhibited preserved bilateral covariance network between homotopic pairs of regions when compared to controls and also that ERK inhibitor treatment did not alter the neuroanatomical organization of scMRI networks (Fig. 4). These findings rule out a contribution of 16p11.2 related genetic alterations in the trophic mechanism underlying scMRI networks.CONCLUSION
We report that prenatal treatment with an ERK inhibitor rescues structural deficits in the ventral hippocampus and lateral septum and rescues anxiety behaviors in 16p11.2del mice5,7. These results provide the first example of the rescue of developmental gray matter abnormalities in this mouse model, and support the translational use of structural MRI to assess putative therapeutic effects in autism.Acknowledgements
A.G. has received funding by the Simons Foundation (SFARI 314688 and 400101)References
[1] Zufferey, F. et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. 49, 660–668 (2012).
[2] Kumar, R. A. et al. Recurrent 16p11.2 microdeletions in autism. Hum. Mol. Genet. 17, 628–638 (2008).
[3] Newbern, J. et al. Mouse and human phenotypes indicate a critical conserved role for ERK2 signaling in neural crest development. Proceedings of the National Academy of Sciences 105, 17115–17120 (2008).
[4] Roskoski, R. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol. Res. 66, 105–143 (2012).
[5] Pucilowska, J. et al. The 16p11.2 deletion mouse model of autism exhibits altered cortical progenitor proliferation and brain cytoarchitecture linked to the ERK MAPK pathway. J. Neurosci. 35, 3190–3200 (2015).
[6] Horev, G. et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proceedings of the National Academy of Sciences 108, 17076–17081 (2011).
[7] Portmann, T. et al. Behavioral abnormalities and circuit defects in the basal ganglia of a mouse model of 16p11. 2 deletion syndrome. Cell reports (2014).
[8] Papale, A. et al. Impairment of cocaine-mediated behaviours in mice by clinically relevant Ras-ERK inhibitors. Elife 5, (2016).
[9] Alexander-Bloch, A et al. Imaging structural co-variance between human brain regions. Nature Reviews Neuroscience 14, 322-336
[10] Cutuli, D. et al. Effects of Omega-3 Fatty Acid Supplementation on Cognitive Functions and Neural Substrates: A Voxel-Based Morphometry Study in Aged Mice. Front Aging Neurosci 8, 38 (2016).
[11] Pagani, M. et al. Semi-automated registration-based anatomical labelling, voxel based morphometry and cortical thickness mapping of the mouse brain. J. Neurosci. Methods 267, 62–73 (2016).
[12] Ullmann, J. F. P. et al. A segmentation protocol and MRI atlas of the C57BL/6J mouse neocortex. Neuroimage 78, 196–203 (2013).
[13] Dorr, A. E., et al. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage (2008).
[14] Pagani, M. et al. Structural covariance networks in the mouse brain. NeuroImage 129, 55-63.
[15] Maren, S. et al. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav. Neurosci. 118, 97–110 (2004).
Figures