2234
A preliminary study on amide proton transfer-weighted MR imaging in patients with obsessive-compulsive disorder1Department of Radiology, Ruijin Hospital,Shanghai Jiao Tong University School of Medicine, Shanghai, People's Republic of China, 2Department of Functional Neurosurgery, Ruijin Hospital,Shanghai Jiao Tong University School of Medicine, Shanghai, People's Republic of China, 3Philips Healthcare, Shanghai, People's Republic of China, 4Philips Healthcare, Cleveland, OH, United States, 5Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 6Department of Psychiatry, Ruijin Hospital,Shanghai Jiao Tong University School of Medicine, Shanghai, People's Republic of China
Synopsis
The aim of this study is to evaluate the feasibility of amide proton transfer-weighted (APTW) MR imaging to detect cerebral abnormalities in patients with obsessive-compulsive disorder (OCD) and to explore its clinical utility. Five OCD patients and 9 normal healthy controls (NC) underwent APTW MR imaging. The magnetic resonance ratio asymmetry (MTRasym) values at 3.5ppm of anterior cingulate cortex (ACC) and thalamus were measured on axial APTW images. We found a trend of increased MTRasym(3.5ppm) or APTW within ACC in OCD patients compared with controls. No significant difference was found between groups in the MTRasym(3.5ppm) within bilateral thalamus. Our results suggest that APTW imaging maybe a promising approach to investigate pathological changes underlying OCD and may provide insights into clinical diagnosis of OCD.
Purpose
Obsessive-compulsive disorder (OCD) is a neuropsychiatric condition characterized by the presence of obsessive thoughts and compulsive behaviors that cause significant distress and impairment [1]. The clinical diagnosis of OCD remains to be based on subjective measurements of symptoms using clinical scales. Amide proton transfer-weighted (APTW) imaging is a novel molecular MRI technique that is able to detect low-concentration endogenous mobile proteins and peptides in tissue which presumably indirectly reflect intracellular metabolic change and physiological and pathological information in vivo [2]. Most current studies of APTW focus on tumors, stroke [3], and neurodegenerative disease [4], whereas there are few studies investigating APTW changes in patients with mental disorders including OCD. The prevailing model of OCD pathophysiology focuses on the cortico-striato-thalamo-cortical circuits, with particular emphasis on the orbitofronto-striato-thalamic (‘affective’) circuits [5, 6]. The anterior cingulate cortex (ACC) and thalamus are key regions in the ‘affective’ circuits. The aim of the present study was to investigate changes in endogenous mobile proteins and peptides in the ACC and thalamus using APTW imaging technique in OCD at 3.0 T MR scanner.Methods
Five OCD patients (age: 29.0±12.0, M/F: 3/2) were recruited from our hospital. Nine gender- and age-matched normal healthy controls (NC) were recruited from the local community by advertisements. All subjects provided written informed consent. MR scanning were performed on a 3.0 T Ingenia MRI scanner (Philips, Best, the Netherlands), using a fifteen-channel head coil. APTW imaging was based on a single-shot, turbo-spin-echo readout sequence with the following parameters: TR=3000 ms, TE=5.6 ms, turbo factor=54, field of view=256 × 256 mm2, matrix size=100 × 100, slice thickness= 4.4mm, acquisition time =192 s. We used four 200ms continuous RF saturation pulses (inter-pulse delay=10 ms, power level=2 μT) and a multi-offset, multi-acquisition APTW imaging protocol. The axial scanning plane was parallel to the anterior commissure-posterior commissure (AC-PC) line, superior to the level of anterior cingulate cortex, and 5 continuous slices were acquired in the inferior-superior direction. T2-weighted fluid-attenuated inversion recovery (FLAIR) images (TR=9000 ms, TE=120 ms, slice thickness=5 mm) were acquired for screening of cerebrovascular diseases and space-occupying lesions in brain. Standard T1 weighted images (TR=2000 ms, TE=20 ms, slice thickness=5 mm) were used as references do draw ROIs manually.
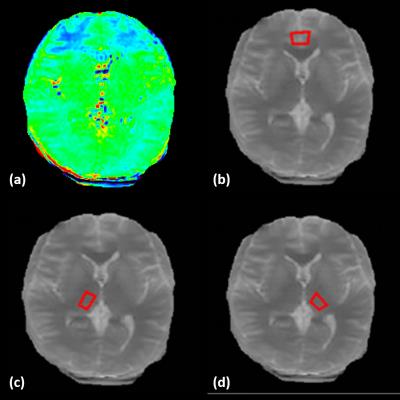
In pseudo-color APTW images, the signals were displayed as red-to-blue in a descending sequence. We drew ROIs on the originally acquired M0: ACC and bilateral thalamus, as shown in Figure 1. Each ROI was carefully drawn and measured three times and the averaged value for each ROI was analyzed for group comparison. We averaged APT values from bilateral hemispheres for both the ACC and thalamus given that there is typically no lateralization for structural changes of OCD onset. The APT value of ROIs was used to reflect the signal intensity of the ROI. Differences in MTRasym(3.5ppm) values between the two groups were analyzed using independent samples t-test analysis.
Results and Discussion
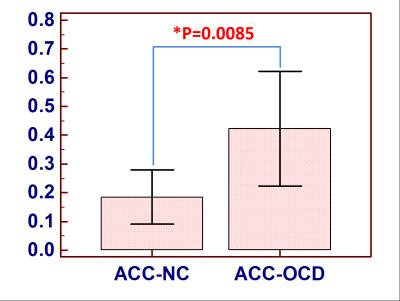
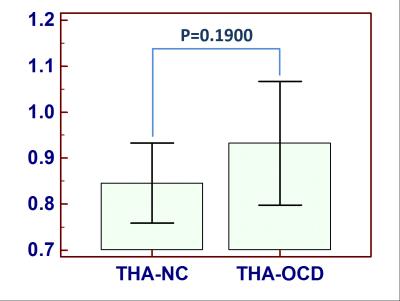
We found that MTRasym(3.5ppm) values of ACC were increased in OCD patients compared to controls (OCD: 0.42±0.16, NC: 0.19±0.12, p=0.0085<0.05, Figure 2). In contrast, there was no statistically significant difference in MTRasym(3.5ppm) within thalamus between groups (OCD: 0.93±0.10, NC:0.85±0.11, P=0.1900>0.05, Figure 3). MTRasym(3.5 ppm) values in ACC in OCD patients were increased. Given that the ACC is intricately connected to the basal ganglia via the cortico-basal ganglia-thalamo-cortical loops, our results are consistent with current theories suggesting that OCD result from an imbalance between the “direct” and “indirect” pathways through the basal ganglia. To our knowledge, this is the first study using APT imaging to investigate changes in endogenous mobile proteins and peptides in OCD. The ACC is involved in numerous cognitive and affective functions, such as detection of conflict, and error monitoring and detection. Several studies have shown both structural and functional abnormalities of the ACC in OCD [5, 7-9]. Interestingly, surgical interventions for refractory OCD such as cingulotomy aim to modulate cortico-striatal-thalamo-cortical (CSTC) activity by targeting ACC regions. Our findings of increased endogenous mobile proteins and peptides of ACC in the patients with OCD partially support the prevailing CSTC models of OCD and offer additional insights. Though the present study is limited by its small sample size, such results indeed indicate the potential application of methods investigating signal changes in patients with OCD.Conclusion
In conclusion, APTW imaging, as a noninvasive MRI method, can show sensitively cerebral abnormal metabolite based on increased proteins and peptides in the ACC in OCD, indicating it may be a promising diagnostic and monitoring tool for OCD. Further studies with larger sample sizes are needed.Acknowledgements
The study was supported in part through State Key Clinical Department of Medical Imaging, and Shanghai Jiao Tong University School of Medicine - Institute of Neuroscience(SHSMU-ION) Research Center for Brain Disorders.References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®): American Psychiatric Publishing.2013.
2. Zhou J, Payen J, Wilson DA, et al. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature Med, 2003; 9(8):1085-1090.
3. Zhou J, Tryggestad E, Wen Z, et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nature Med, 2011; 17(1): 130-134.
4. Li C, Peng S, Wang R, et al. Chemical exchange saturation transfer MR imaging of Parkinson's disease at 3 Tesla. Eur Radiol, 2014; 24:2631-9.
5. Menzies L, Chamberlain S R, Laird A R, et al. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatal model revisited. Neuroscience & Biobehavioral Reviews, 2008, 32(3):525-549.
6. Milad M R, Rauch S L. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends in Cognitive Sciences, 2012, 16(1):43-51.
7. Fitzgerald K D, Stern E R, Angstadt M, et al. Altered function and connectivity of the medial frontal cortex in pediatric obsessive compulsive disorder. Biological Psychiatry, 2010, 68(11):1039-47.
8. Schlösser R G M, Wagner G, Schachtzabel C, et al. Fronto-cingulate effective connectivity in obsessive compulsive disorder: A study with fMRI and dynamic causal modeling. Human Brain Mapping, 2010, 31(12):1834-1850.
9. de Wit S J, Alonso P, Schweren L, et al. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. American Journal of Psychiatry, 2013, 171(3):340-349.
Figures