2233
Homozygous loss of autism-risk gene CNTNAP2 results in reduced local and long-range prefrontal connectivity1CIMeC, Center for Mind/Brain Sciences, University of Trento, Rovereto, Italy, 2Functional Neuroimaging Laboratory, Center for Neuroscience and Cognitive Systems @ UniTn, Istituto Italiano di Tecnologia, Rovereto, Italy, 3Neurotoxicology and Neuroendocrinology Section, Department of Cell Biology and Neurosciences, Istituto Superiore di Sanità, Rome, Italy, 4Neural Computation Laboratory, Center for Neuroscience and Cognitive Systems @ UniTn, Istituto Italiano di Tecnologia, Rovereto, Italy
Synopsis
Functional connectivity aberrancies as measured with resting-state fMRI (rsfMRI) have been consistently observed in the brains of autism spectrum disorders (ASD) patients. However, genetic and neurobiological underpinnings of these findings remain unclear. Here we used rsfMRI to show that homozygous mice lacking the strongly ASD-associated gene CNTNAP2 exhibit default-mode network connectivity alterations associated with reduced social investigation, a core “autism trait” in mice. These findings reveal a causal link between an ASD-associated mutation and functional connectivity aberrancies and suggest that homozygous loss-of-function mutations in CNTNAP2 may predispose to ASD through a selective dysregulation of functional coupling between integrative cortical areas.
Background
Neuroimaging studies have consistently revealed atypical functional connectivity, as measured via temporal covariance in resting-state fMRI (rsfMRI) BOLD signal, across brain regions of autistic spectrum disorders (ASD) patients1. These findings have led to the hypothesis that aberrant functional coupling might represent a common pathway or pathogenetic correlate of the autistic phenotype to which different ASD etiologies converge2. However, the neurophysiological and genetic underpinnings of these connectional derangements remain unknown. The recent optimization of rsfMRI in rodents3 offers the opportunity to investigate the neurobiological foundations of aberrant connectivity seen in ASD4 via imaging studies in mouse lines recapitulating high-confidence ASD mutations5.
Homozygous loss-of-function mutations in Contactin Associated Protein-like 2 (CNTNAP2) encoding CASPR2, a neurexin-related cell-adhesion molecule, are strongly linked to autism and epilepsy6–8 and lead to abnormal neuronal migration, reduction in GABAergic neurons and autism-like behavioural traits in the mouse consistent with ASD symptoms in humans9. Common genetic variants in CNTNAP2 have also been associated with impaired frontal lobe functional connectivity in humans10. However, a causal relationship between loss-of-function mutations in CNTNAP2 and functional connectivity remains to be established, and the role of CNTNAP2 in shaping macroscale circuit assembly, together with the specific substrates affected, remain unclear.
Methods
All experiments were carried out in accordance with Italian regulations governing animal welfare and protection. We used BOLD rsfMRI and diffusion-weighted (DW) MRI to map large-scale functional connectivity and white matter topology in three-month old male Cntnap2-/- (N=13) and wild-type Cntnap2+/+ (N=13) mice. We additionally performed a series of social interaction and investigation behavioural tests on the same experimental cohort11,12.
Resting-state fMRI. Mice were imaged under shallow halothane anaesthesia (0.75%) and artificial ventilation13,14 on a 7T MRI scanner using a single-shot EPI sequence (TR/TE 1200/15 ms, flip angle 30°, FOV 2 × 2 cm2, matrix 100 × 100, 24 coronal slices, slice thickness 0.50 mm, 500 volumes) and the data was preprocessed as previously described15. To identify foci of connectivity disruptions, we calculated global and local brain connectivity maps16,17 for all subjects and mapped voxelwise inter-group differences in both measures. We additionally mapped anteroposterior DMN connectivity between a prelimbic and a series of cingulate seeds.
Diffusion MRI. Ex vivo diffusion-weighted (DW) MRI was carried out on paraformaldehyde fixed specimens18. Each DW dataset was composed of 8 B0 images and 81 diffusion gradient-encoding directions with b=3000 s/mm2 (∂=6 ms, ∆=13 ms) acquired using an EPI sequence (TR/TE=13500/27.6 ms, FOV 1.68 × 1.54 cm2, matrix 120 × 110, in-plane spatial resolution 140 × 140 µm2, 54 coronal slices, slice thickness 280 µm, 20 averages). Whole brain tractography was performed with MRtrix319 using constrained spherical deconvolution20 (lmax = 8) and probabilistic tracking (iFOD221).
Results and discussion
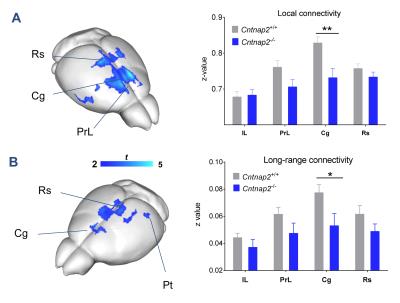
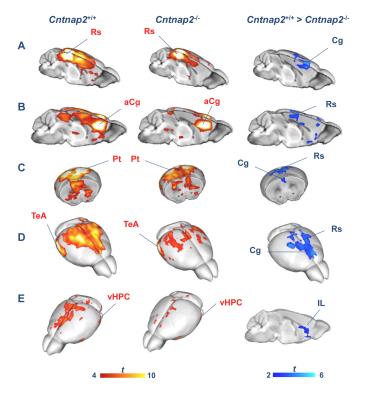
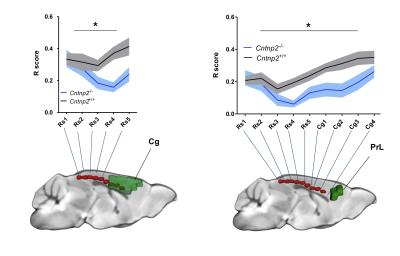
We observed foci of significantly reduced local and long-range connectivity in Cntnap2-/- mutants with respect to wild-type control subjects encompassing prefrontal (prelimbic and cingulate) and retrosplenial cortices (Fig. 1). Interestingly, these same brain regions have been classified both in mice and in humans as functional hubs due to their high connection strength16,23, and as such are thought to play a key integrative contribution in distributed functional networks. Furthermore, a clear disconnection between posterior (retrosplenial) and middle/anterior portions of the DMN (cingulate, prelimbic cortex) was apparent (Fig. 2, 3).
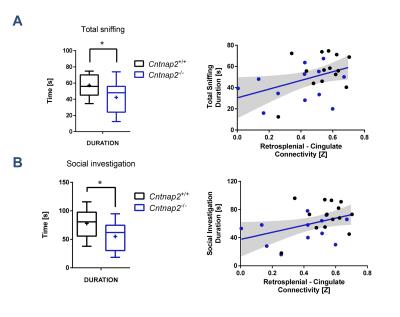
Consistent with previous studies9, behavioural testing revealed significantly impaired social interest and increased non-social behaviour in Cntnap2-/- mutants compared to Cntnap2+/+ control littermates (Fig. 4, left). Interestingly, the hypoconnectivity between key DMN components (retrosplenial-cingulate cortex) was significantly associated with reduced social behaviour and increased non-social behaviour (Fig. 4, right), recapitulating similar findings in human imaging studies22. These findings suggest that the observed impairment in antero-posterior functional coupling could affect complex behavioural traits such as sociability and social exploration.
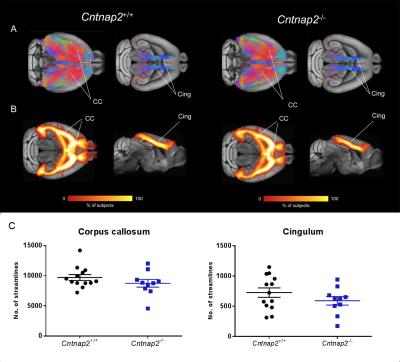
To further probe a role of macroscale anatomical connectivity on the observed functional decoupling in Cntnap2-/-, we used whole-brain tractography to dissect two major white matter tracts characterised by extensive cortico-cortical antero-posterior extension24: corpus callosum and cingulum tracts. These tracts appeared to be largely typical in Cntnap2-/- mutants (Fig. 5).
Conclusions
In conclusion, we document that absence of CNTNAP2 leads to local and long-range connectivity reductions in prefrontal functional hubs of the mouse brain, an effect associated with impaired social behaviour. The lack of white-matter connectional alterations at the macroscale level suggests that the observed functional connectivity alterations reflect functional decoupling, rather than major anatomical re-wiring. Collectively, these findings suggest that loss-of-function mutations in CNTNAP2 may predispose to neurodevelopmental disorders and autism through dysregulation of macroscale functional network couplings, and provide a translational model for investigating connectional perturbations in syndromic ASD forms.Acknowledgements
The study was funded by a grant from the Simons Foundation
(SFARI 314688 and 400101, A.G.).
References
1. Ecker C, Bookheimer SY, Murphy DGM. Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. The Lancet. Neurology. 2015;14(11):1121–1134.
2. Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neuroscience and Biobehavioral Reviews. 2012;36(4):1292–1313.
3. Gozzi A, Schwarz AJ. Large-scale functional connectivity networks in the rodent brain. NeuroImage. 2016;127:496–509.
4. Liska A, Gozzi A. Can mouse imaging studies bring order to autism connectivity chaos? Frontiers in Neuroscience. 2016;10:484.
5. Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, Murtha MT, Bal VH, Bishop SL, Dong S, et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron. 2015;87(6):1215–1233.
6. Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. American Journal of Human Genetics. 2008;82(1):150–159.
7. Rodenas-Cuadrado P, Ho J, Vernes SC. Shining a light on CNTNAP2: complex functions to complex disorders. European journal of human genetics: EJHG. 2014;22(2):171–178.
8. Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. The New England Journal of Medicine. 2006;354(13):1370–1377.
9. Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147(1):235–246.
10. Scott-Van Zeeland AA, Abrahams BS, Alvarez-Retuerto AI, Sonnenblick LI, Rudie JD, Ghahremani D, Mumford JA, Poldrack RA, Dapretto M, Geschwind DH, et al. Altered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2. Science Translational Medicine. 2010;2(56):56ra80.
11. Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes, Brain, and Behavior. 2011;10(1):44–56.
12. Scattoni ML, Martire A, Cartocci G, Ferrante A, Ricceri L. Reduced social interaction, behavioural flexibility and BDNF signalling in the BTBR T+ tf/J strain, a mouse model of autism. Behavioural Brain Research. 2013;251:35–40.
13. Ferrari L, Turrini G, Crestan V, Bertani S, Cristofori P, Bifone A, Gozzi A. A robust experimental protocol for pharmacological fMRI in rats and mice. Journal of Neuroscience Methods. 2012;204(1):9–18.
14. Sforazzini F, Bertero A, Dodero L, David G, Galbusera A, Scattoni ML, Pasqualetti M, Gozzi A. Altered functional connectivity networks in acallosal and socially impaired BTBR mice. Brain Structure & Function. 2016;221(2):941–954.
15. Sforazzini F, Schwarz AJ, Galbusera A, Bifone A, Gozzi A. Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. NeuroImage. 2014;87:403–415.
16. Liska A, Galbusera A, Schwarz AJ, Gozzi A. Functional connectivity hubs of the mouse brain. NeuroImage. 2015;115:281–291.
17. Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. NeuroImage. 2010;49(4):3132–3148.
18. Dodero L, Damiano M, Galbusera A, Bifone A, Tsaftsaris SA, Scattoni ML, Gozzi A. Neuroimaging evidence of major morpho-anatomical and functional abnormalities in the BTBR T+TF/J mouse model of autism. PloS One. 2013;8(10):e76655.
19. Tournier J-D, Calamante F, Connelly A. MRtrix: Diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology. 2012;22(1):53–66.
20. Tournier J-D, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. NeuroImage. 2007;35(4):1459–1472.
21. Tournier J-D, Calamante F, Connelly A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. In: Proceedings of the International Society for Magnetic Resonance in Medicine (ISMRM). Vol. 18. 2010. p. 1670.
22. Schreiner MJ, Karlsgodt KH, Uddin LQ, Chow C, Congdon E, Jalbrzikowski M, Bearden CE. Default mode network connectivity and reciprocal social behavior in 22q11.2 deletion syndrome. Social Cognitive and Affective Neuroscience. 2014;9(9):1261–1267.
23. Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(6):1860–1873.
24. Vogt BA, Paxinos G. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Structure & Function. 2014;219(1):185–192.
Figures