2226
Molecular imaging of pediatric cerebellar tumors using endogenous protein-based amide proton transfer MR imaging at 3 Tesla1Department of Radiology, Beijing Children’s Hospital, Capital Medical University, Beijing, People's Republic of China, 2Department of Radiology, Johns Hopkins University, MD, United States
Synopsis
Amide proton transfer (APT)-MRI is a chemical exchange saturation transfer (CEST) based approach in which the amide protons of endogenous proteins and peptides are irradiated to accomplish detection using the water signal. In this study, the APT approach was incorporated with standard brain MRI sequences and applied to children with cerebellar tumors at 3T. The initial results show that APT imaging can enhance the noninvasive identification of tissue heterogeneity and may be a biomarker of tumor grade in pediatric cerebellar tumors.
Introduction/Purpose
Brain tumors are the most prevalent solid tumors in children. There are currently more than 4600 tumors of the central nervous system diagnosed annually in the United States in children, corresponding to an incidence rate of 5.57 per 100,000. 1 In children older than 1 year of age, most tumors are located within the posterior fossa. Conventional magnetic resonance imaging (MRI) is essential for diagnosis as well as for the evaluation of primary location (gray vs. white matter), quality (solid vs. cystic), tumor extent, and involvement of eloquent brain regions but often offers limited information regarding tumor type/histology and grade. 2 Amide proton transfer (APT) imaging3,4 is a new molecular-MRI technique that detects endogenous mobile proteins and peptides in tissue via saturation of the amide protons in the peptide bonds. The purpose of this abstract is to demonstrate that APT can potentially provide information about the heterogeneity and grading of pediatric cerebellar tumors based on increased cellular content of proteins and peptides.Materials and Methods
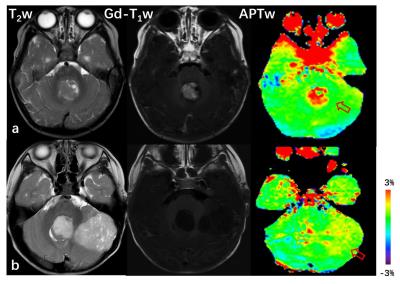
Eight children with cerebellar tumor [three with pilocytic astrocytoma (PA), two with medulloblastoma (MB), one with ependymoma, one with haemangioblastoma, and one with atypical teratoid/rhabdoid tumor (AT/RT)] were recruited in this study. MRI data was acquired using a Philips 3T MRI scanner, including multiple MRI scans, T1-weighted, T2-weighted, isotropic apparent diffusion constant (ADC), Gd-T1w, and APT-weighted. Gd T1w was the last sequence acquired. APTw MR imaging was based on the single-slice, single-shot TSE (saturation time = 800 ms; saturation power = 2 μT). The APT effect was quantified using an MT-ratio asymmetry analysis at the offset of 3.5 ppm: MTRasym(3.5ppm), and displayed using a window of -3% to 3%. The Gd-T1w image was used as the reference of ROI analysis.Results and Discussion
Fig. 1 shows an example of the APTw and standard MR images for patients with WHO grade-III/IV MB(a) and WHO grade-I/II PA (b). High-grade MB demonstrates significant gadolinium enhancement. There is a large APTw intensity increase in the tumor area compared to normal-appearing cerebellar tissue. The area of the highest intensity on APTw corresponds well to the area of maximal Gd-enhancement on T1w with decreasing intensity in the non-enhancing portion (likely necrosis) of the tumor core. PA shows no enhancement on Gd-T1w and intermediate to slightly hyperintensity on APTw. which may be characteristic of low-grade tumor. The APTw hyperintensity is a typical feature of the tumor, suggesting increased content of mobile proteins and peptides.5,6 Further, regions of increased APTw extend outside of the tumor core (signified by Gd-enhancement) and into the peripheral brain zones (likely tumor infiltration, which could not be identified by Gd-MRI).
Our initial data show that APTw intensity is consistently higher in the tumor core than in normal-appearing cerebellar tissue (two-tailed paired Student’s t-test, 2.4±0.3 vs. 0.5±0.08, p < 0.001). APTw intensity of high-grade cerebellar tumors is higher than low-grade cerebellar tumors (independent sample Student’s t-test, 2.6±0.4 vs.1.5±0.1, p < 0.05).Thus, APT may help better distinguish high- and low-grade cerebellar tumors presurgically to assist with treatment planning by providing targets for tissue sampling to yield the most accurate diagnosis.
Conclusions
Early results suggest that APT imaging may allow for better characterization and grading of pediatric cerebellar tumors noninvasively without the need of exogenous contrast agents. These unique capabilities of APT imaging may be very important for increasing diagnostic accuracy and safety of MRI for human brain tumors. This is particularly significant for pediatric cases, where intravenous access is often problematic.Acknowledgements
Dr. Jinyuan Zhou is a co-inventor on a patent at the USA Patent and Trademark Office for the APT-MRI technology that is licensed to Philips Healthcare. This patent is owned and managed by Johns Hopkins University. This study has received funding from theNational Natural Science Foundation of China (31271161) and from theNational Institutes of Health (R01NS083425, R01EB009731, andR01CA166171).References
1.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol. 2015. 17 Suppl 4: iv1-iv62.
2.Poretti A, Meoded A, Huisman TA, et al.Neuroimaging of pediatric posterior fossa tumors including review of the literature. J Magn Reson Imaging. 2012. 35(1): 32-47.
3.Jones CK, Schlosser MJ, van Zijl PC, et al.Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med. 2006. 56(3): 585-92.
4.Wen Z, Hu S, Huang F, et al. MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage. 2010. 51(2): 616-22.
5.Hobbs SK, Shi G, Homer R, et al. Magnetic resonance image-guided proteomics of human glioblastoma multiforme. J Magn Reson Imaging. 2003. 18(5): 530-6.
6.Howe FA, Barton SJ, Cudlip SA, et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2003. 49(2): 223-32.