2224
Development and validation of a MRI-based radiomics prognostic classifier in patients with primary glioblastoma multiforme1Department of radiology, Guangzhou First People's Hospital, Guangzhou, People's Republic of China, 2Department of radiology, Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou, People's Republic of China, 3GE Healthcare China, Guangzhou, People's Republic of China
Synopsis
Glioblastoma multiforme (GBM) is the most frequent and deadly type of primary malignant tumor in the central nervous system with a median survival range of only 9-15 months. We developed and validated a MRI-based radiomics classifier to predict overall survival(OS) in patients with newly diagnosed GBM. The results showed that a 7-radiomics classifier allows prediction of survival and stratification of patients with newly diagnosed GBM into a low- or high-risk group for OS.
Introduction
Glioblastoma multiforme
(GBM) is the most frequent and deadly type of primary malignant tumor in the
central nervous system. Standard treatment consists of maximal surgical
resection followed by adjuvant chemotherapy and radiation therapy, which could
improve the median survival time of GBM from 15.3 months to 21.7 months.
However the survival time varies widely from 3 months to 3 years after diagnosis1, which underscored the limitations of current
clinicopathologic markers and grading systems in predicting patient prognosis.
Therefore, this study aimed to develop and validate a MRI-based radiomics classifier
to predict overall survival(OS) in patients with newly diagnosed GBM.Methods
Clinical and MR imaging
data of 127 patients with GBM were obtained from the Cancer Genome Atlas and
the Cancer Imaging Archive. Regions of interest(ROIs) corresponding to enhanced
portions of tumor were drawn on the post-contrast T1 weighted images of 127
patients (allocated in a 2:1 ratio to a training[n=85] or validation[n=42]
set), and a total of 3824 radiomics features including first-order and texture
features were derived from these ROIs. Radiomics features of patients in the
training set were subjected to the minimum redundancy maximum relevance (mRMR)
algorithm and Cox proportional hazard regression model to predict OS. The performance of a cox proportional hazards model with the
Akaike’s information criterion(AIC)
criteria was assessed with concordance index(C-index).Results
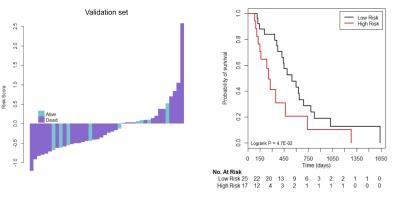
Seven radiomics features were selected as a classifier in the training set could stratify patients with GBM into a low- or high-risk group for OS(HR, 3.089, 95% CI,1.793-5.322; P<0.001), and the results were validated successfully in the validation set(HR, 2.065, 95%CI, 0.996-4.281; P<0.001)(Figure1). The performance of the radiomics classifier in training set(C-index, 0.790, 95%CI, 0.684-0.896) was similar to that in validation set(C-index, 0.737,95% CI:0.566-0.908).Discussion
Many findings of previous studies have demonstrated that major differences in phenotypes or gene-protein classifiers can be correlated to radiographic findings2,3. And a recent literature on the patterns of genomic instability in metastatic pancreatic cancer showed that tumors with more genomic heterogeneity were more likely to have a worse prognosis4. Accordingly, tumor heterogeneity assessed through imaging could be translated to an expression of genomic heterogeneity and would exhibit poor prognosis. The 7 radiomics features selected for the radiomics classifier derived from the enhanced portion of tumor in this study are in consistent with the recent researches on risk stratification of survival outcomes of patients5. While further studies on a causal relationship between gene expression and image features were needed to be extended.Conclusion
A 7-radiomics classifier allows prediction of survival and stratification of patients with newly diagnosed GBM.Acknowledgements
Zhongping Zhang is an employee of GE Healthcare, China, but does not have any conflict of interest with this project.References
1. Hegi ME, Diserens AC, Gorlia T, et al. mgmt gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352 (10): 997-1003
2. Lambin P, Rios-Velazquez E, Leijenaar R, et al. radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(4):441-446
3. Hobbs SK, Shi G, Homer R. et al. Magnetic resonance image-guided proteomics of human glioblastoma multiforme. J Magn Reson Imaging. 2003;18(5): 530-536
4. Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467(7319): 1109-1113
5. Kickingereder P, Burth S, Wick A, et al. Radiomic Profiling of Glioblastoma: Identifying an Imaging Predictor of Patient Survival with Improved Performance over Established Clinical and Radiologic Risk Models. Radiology. 2016;280(3): 880-889
Figures