2221
Differentiation of Brain Infections from Necrotic Glioblastomas using Diffusion Tensor Imaging and Perfusion Weighted Imaging1Radiology, UNIVERSITY OF PENNSYLVANIA, PHILADELPHIA, PA, United States, 2Biological Anthropology, National Research Centre, Cairo, Egypt, 3Cellular and Molecular Physiology, University of Liverpool, Liverpool, United Kingdom
Synopsis
To determine whether combined use of diffusion and perfusion MRI can help in differentiating brain infections from glioblastomas (GBMs), 13 patients with infections and 20 patients with GBMs underwent DTI and DSC-PWI. Significantly lower median values of mean diffusivity (MD) and higher fractional anisotropy (FA) were observed in central core of infective lesions than in GBMs. Additionally, significantly decreased median and maximum values of relative cerebral blood volume (rCBV) from enhancing region were observed in patients with infections compared with those of GBMs. The best classification model consisted of MD from central core and rCBVmax from enhancing region.
T
Purpose: Differentiation of brain infections from glioblastomas (GBMs) is crucial as prognosis and clinical management of these patients is quite different.1 Using diffusion tensor imaging (DTI), few studies2-4 have reported the differentiation of these two entities. However, high diffusivity similar to that found in necrotic high-grade gliomas has been reported in 5-21% of untreated abscesses.5 Moreover, infective lesions may also demonstrate high anisotropy mimicking GBMs6 suggesting that differential diagnosis may not always be possible on the basis of DTI alone. Dynamic susceptibility contrast perfusion weighted imaging (DSC-PWI) derived cerebral blood volume (CBV) has been used to differentiate infective from neoplastic lesions but with conflicting results.7,8 Some have reported similar patterns of CBV in these two lesions, while others have found a significant decrease in CBV in infective lesions compared to neoplastic lesions. Given that DTI and DSC-PWI provide different physiological information, we hypothesize that synergistic interactions between DTI parameters and CBV may improve distinction of these two conditions. This study aims to investigate the potential of combined use of DTI and DSC-PWI to distinguish brain infections from GBMs.
Methods: A cohort of 13 patients (8M/5F, mean age=45.7±12.2years) with brain infections and 20 patients (10M/10F, mean age 60±12.2 years) with GBMs underwent anatomical imaging, DTI and PWI on a 3T MR system. DTI data were acquired using 30 directions with a single-shot spin-echo EPI with parallel imaging (acceleration factor=2), TR/TE=5000/86ms; NEX=3; in-plane resolution=1.72 × 1.72mm2, slice thickness=3mm, b= 0, 1000s/mm2. For DSC-PWI, T2* weighted gradient-echo EPI sequence was acquired using the following parameters: TR/TE = 2000/45ms, in-plane resolution=1.72 × 1.72mm2, 20 slices with thickness= 3 mm. Forty-five sequential measurements were acquired for each section, with a temporal resolution of 2.1s. After motion and eddy current correction of raw DTI data, mean diffusivity (MD) and fractional anisotropy (FA) were computed using in-house developed algorithm.9,10 Leakage corrected CBV maps were constructed using NordicICE program. Contrast-enhanced T1 weighted images, FLAIR, CBV and DTI maps were co-registered and a semi-automated segmentation routine was used to segment the lesion into enhancing (ER) and central regions (CR). The median MD, FA, and relative CBV (rCBV) were computed from these regions. The 90th percentile rCBV values were also measured from enhancing region to compute maximum rCBV (rCBVmax). These parameters were compared between brain infections and GBMs using Mann-Whitney-U tests. Receiver operating characteristic (ROC) curve and multivariate logistic regression analyses were performed to determine the best model for classification.
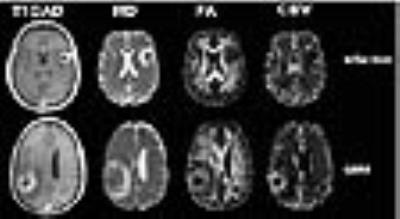
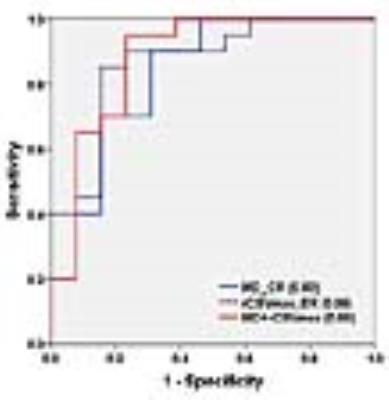
Results: Representative anatomical images, MD, FA and rCBV maps from a patient each from GBM and brain infection is shown in Figure 1. Significantly lower MD was observed from the central core in patients with infections compared to those of GBMs. Also, significantly decreased median and maximum values of relative cerebral blood volume (rCBV) from enhancing region were observed in patients with infections compared with those of GBMs. A pair-wise comparison of DTI parameters and rCBVmax is shown in Box-Whisker plots (Fig. 2). Using ROC and multivariate logistic regression analyses, the best model to distinguish brain infections from GBM consisted of MD from central core, rCBVmax from enhancing region resulting in area under the ROC curve (AUC) of 0.88 (Fig. 3). This classification model provided a sensitivity of 95% and specificity of 77% in distinguishing brain infections from GBMs.
Discussion: Our results indicate that different pattern water diffusion and hemodynamics may allow discrimination of brain abscess from GBMs with high sensitivity and specificity. In consistent with previous report,6 significantly lower median values of MD and higher FA were observed in central core of infective lesions than in GBMs. These findings suggest that central core of infections and GBMs may harbor differential degrees of cellularity, viscosity and cyto-architectural composition (number of inflammatory cells, protein molecules, and arrangement of collagen fibers).11 We also observed significantly higher median rCBV and rCBVmax in GBMs than in infective lesions from enhancing regions of lesions secondary to angiogenesis of viable neoplastic cells and increased expression of vascular endothelial growth factors and increased microvascular density with the tumor bed.12
Conclusions: We believe that DTI and DSC-PWI provide complementary physiological information that may improve characterization and differentiation of brain infections from GBMs with high sensitivity and specificity.
Acknowledgements
No acknowledgement found.References
1. Alvis MH, Castellar-Leones SM, Elzain MA, et al. J Neurosci Rural Pract. 2013;4(Suppl 1):S67-81.
2. Reiche W, Schuchardt V, Hagen T, et al. Differential diagnosis of intracranial ring enhancing cystic mass lesions: role of diffusion-weighted imaging (DWI) and diffusion-tensor imaging (DTI). Clin Neurol Neurosurg. 2010; 112: 218-225.
3. Toh CH, Wei KC, Ng SH et al. Differentiation of brain abscesses from necrotic glioblastomas and cystic metastatic brain tumors with diffusion tensor imaging. Am J Neuroradiol. 2011;32(9):1646-1651.
4. Nath K, Agarwal M, Ramola M, et al. Role of diffusion tensor imaging metrics and in vivo proton magnetic resonance spectroscopy in the differential diagnosis of cystic intracranial mass lesions. Magn Reson Imaging. 2009;27(2):198-206.
5. Lee EJ, Ahn KJ, Ha YS, et al. Unusual findings in cerebral abscess: report of two cases. Br J Radiol. 2006;79: e156-161.
6. Tung GA, Evangelista P, Rogg JM, et al. Diffusion-weighted MR imaging of rim-enhancing brain masses: is markedly decreased water diffusion specific for brain abscess? Am J Roentgenol. 2001;177: 709-712.
7. Holmes TM, Petrella JR, Provenzale JM. Distinction between cerebral abscesses and high-grade neoplasms by dynamics susceptibility contrast perfusion MRI. Am J Neuroradiol. 2004;183:1247-1252.
8. Chan JH, Tsui EY, Chau LF, et al. Discrimination of an infected brain tumor by cerebral brain abscess by combined MR perfusion and diffusion imaging. Comput Med Imaging Graph. 2002;26:19-23.
9. Wang S, Kim S, Chawla S, et al. Differentiation between glioblastomas and solitary brain metastases using diffusion tensor imaging. Neuroimage. 2009;44(3):653-660.
10. Wang S, Kim S, Chawla S, et al. Differentiation between glioblastomas, solitary brain metastases, and primary cerebral lymphomas using diffusion tensor and dynamic susceptibility contrast-enhanced MR imaging. Am J Neuroradiol. 2011;32(3):507-514.
11. Gupta RK, Hasan KM, Mishra AM, et al. High fractional anisotropy in brain abscesses versus other cystic intracranial lesions. Am J Neuroradiol. 2005;26(5):1107-1114.
12. Haris M, Gupta RK, Singh A et al. Differentiation of infective from neoplastic brain lesions by dynamic contrast-enhanced MRI. Neuroradiology. 2008;50(6):531-540.
Figures