2213
Relating the Evolution of RT-Induced Vascular Injury to surrounding white matter microstructure in Adult Glioma Patients1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States
Synopsis
In the treatment of gliomas, radiation therapy (RT) is associated with long-term effects including vascular injury in the form of cerebral microbleeds (CMBs), and changes in the white matter and thickness of cortex. Ultra high-field MRI techniques were used to characterize RT-induced changes across serial scans in support of ongoing investigations of the role of anti-angiogenic therapies in minimizing treatment effects. Steady increases in the number of CMBs was observed decades post-RT. CMB foci were characterized by increased isotropic diffusion when compared to surrounding white matter, which showed signs of degradation in as few as two months between serial scans.
Purpose
While radiation therapy (RT) remains a standard practice in the upfront treatment of high-grade gliomas and low grade gliomas at the time of recurrence, it is often associated with long-term side effects including vascular injury and cognitive decline.1 With current treatment strategies, survival for WHO (world health organization) grades II and III gliomas is between 6 and 10 years,2 thus measures of quality-of-life (QOL) serve as a relevant endpoint for these patients who live substantially longer than patients with glioblastoma. Towards maximizing patient QOL, a co-primary goal next to survival should be to minimize the deficits incurred through treatment. RT-induced vascular injury typically manifests as size-varying hemosiderin deposits in the brain called cerebral microbleeds (CMBs), which are capable of being detected with Susceptibility-Weighted Imaging (SWI) as early as 8 months following treatment.3 Usually accompanying CMBs are changes in the integrity of the white matter (WM) pathways and thickness of the cortex.4,5 While it has been shown that the number of CMBs progressively increases, and that the WM degrades and cortex thins following treatment, there is scant data evaluating both global and regional changes simultaneously, or at ultra-high field strengths where visualization is enhanced6. Understanding the chronology of RT-induced changes in the brain, and more thoroughly characterizing serial changes in the distribution and density of CMBs, will support ongoing work investigating the use of anti-angiogenic or other radiosensitizing agents to alter RT-induced processes leading to injury.3Methods
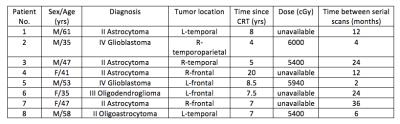
Thus far, data have been evaluated for eight of the eighteen (5 male, 3 female; mean age 47.1 ± 9.8) who were treated with RT approximately 4 to 20 years’ prior for a glioma brain tumor (Figure 1). Patients were scanned on a 7T scanner with a 32 channel receive coil. High-resolution SWI data, 2-shell, 24- and 55-directional DTI, and T1-weighted images of brain anatomy were acquired across two serial scans. The time between scans varied across patients from 2 months up to 3 years. CMBs were detected using an in-house automated CMB detection algorithm7,8, recently updated to perform CMB volume quantification9. The number and volume of CMBs were quantified across serial scans; between-patient comparisons were made with respect to radiation dose, time since RT, and time between serial scans. Diffusion parameters, including fractional anisotropy (FA), mean, longitudinal, and radial diffusivity (MD, LD, RD) were evaluated at the CMB foci as well as within the surrounding WM. Mean values for the CMB foci and WM were contrasted, and assessed across serial scans. The orientation dispersion index (ODI) and free water volume fraction (FISO) from the NODDI (neurite orientation dispersion and density imaging) toolbox were evaluated in the same manner. Tissue contrast on the T1-weighted anatomical images was first corrected with an optimized N4 bias field correction algorithm, then segmented with FreeSurfer (version 5.3.0) to quantify cortical thickness. Regional changes in cortical thickness were assessed, and associated with CMB characteristics.Results
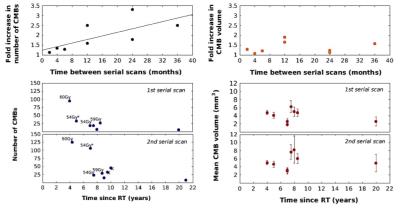
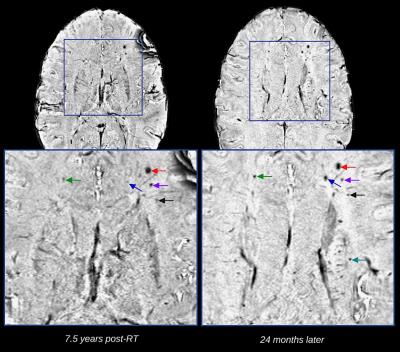
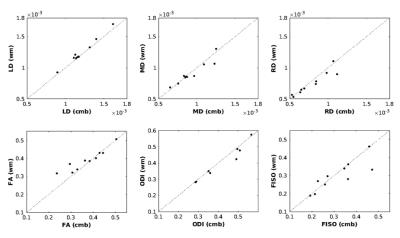
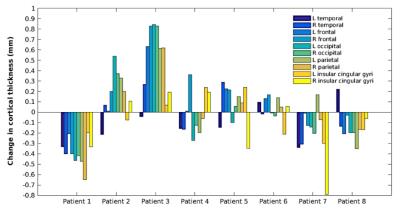
The number and mean volume of CMBs ranged between 6 and 125 CMBs, and 1.87 and 8.24 mm3 respectively, but were uncorrelated with time since CRT (Figure 2). The influence of radiation dose is displayed in Figure 2 for two patients treated 4 and 5 years prior with 60Gy and 54Gy, respectively. The fold increase in CMBs, was positively correlated with time between serial scans. A weaker trend held for the fold increase in mean volume of CMBs, however, there were observable changes in CMB volumes overtime (Figure 3). When compared with the surrounding WM, CMB foci were predominately characterized by decreased LD and FA, and increased MD, RD, ODI and FISO (Figure 4). Identical trends were observed in the surrounding WM across serial scans. Regional changes in cortical thickness varied between patients, with no direct association to tumor location where the majority of CMBs were detected.Discussion/Conclusion
Preliminary findings here suggest that there is a steady increase in the number of CMBs even decades following treatment, and confirms that radiation dose is a determinate of CMB evolution. Diffusion parameters can be used to characterize CMB foci, demonstrating increased isotropic diffusion and structural degradation over the surrounding WM. Serial changes in these parameters can be observed in as few as two months between scans. Data from the present cohort, augmented by added serial scans and data from the remaining 10 patients, will enable a more thorough investigation of these RT-induced changes. This includes the rate of CMB growth, refining the relationship between dose and CMB presence, evaluating changes in the distribution of CMBs according to dose maps, and relating CMB characteristics to structural and functional connectivity.Acknowledgements
The study was supported by NIH grant RO1HD079568 and a GE Healthcare investigator initiated grant.References
1. Greene-Schloesser, D., Robbins, M. E., Peiffer, A. M., Shaw, E. G., Wheeler, K. T., & Chan, M. D. (2012). Radiation-induced brain injury: A review. Frontiers in Oncology, 2(July), 73. http://doi.org/10.3389/fonc.2012.00073
2. Sanai, N., Chang, S., & Berger, M. S. (2011). Low-grade gliomas in adults. J Neurosurg, 115, 948–65. http://doi.org/10.3171/2011.7.JNS10238
3. Lupo, J. M., Molinaro, A. M., Essock-Burns, E., Butowski, N., Chang, S. M., Cha, S., & Nelson, S. J. (2016). The effects of anti-angiogenic therapy on the formation of radiation-induced microbleeds in normal brain tissue of patients with glioma. Neuro-Oncology, 18(1), 87–95. http://doi.org/10.1093/neuonc/nov128
4. Valk, P. E., & Dillon, W. P. (1991). Radiation injury of the brain. American Journal of Neuroradiology, 12(1), 45–62
5. Karunamuni, R., Bartsch, H., White, N.S., Moiseenko, V., Carmona, R., Marshall, D.C., Seibert, T.M., McDonald, C.R., Farid, N., Krishnan, A., Kuperman, J., Mell, L., Brewer, J.B., Dale, A.M., Hattangadi-Gluth, J.A. (2016). Dose-Dependent Cortical Thinning After Partial Brain Irradiation in High-Grade Glioma. Int J Radiat Oncol Biol Phys, 94(2): 297-304. http://doi.org/10.1016/j.ijrobp.2015.10.026
6. Lupo, J. M., Li, Y., Hess, C. P., & Nelson, S. J. (2011). Advances in ultra-high field MRI for the clinical management of patients with brain tumors. Current Opinion in Neurology, 24(6), 605–15. http://doi.org/10.1097/WCO.0b013e32834cd495
7. Bian, W., Banerjee, S., Kelly, D. A. C., Hess, C. P., Larson, P. E. Z., Chang, S. M., Lupo, J. M. (2015). Simultaneous imaging of radiation-induced cerebral microbleeds, arteries and veins, using a multiple gradient echo sequence at 7 Tesla. Journal of Magnetic Resonance Imaging, 42(2), 269–279. http://doi.org/10.1002/jmri.24802
8. Bian, W., Hess, C. P., Chang, S. M., Nelson, S. J., & Lupo, J. M. (2013). Computer-aided detection of radiation-induced cerebral microbleeds on susceptibility-weighted MR images. NeuroImage: Clinical, 2(1), 282–290. http://doi.org/10.1016/j.nicl.2013.01.012
9. Zou, X., Wei., B, Lafontaine, M., Hess, C.P., Nelson, S.J., Lupo, J.M. (2015). Automated 3-D Segmentation of Radiation-induced Cerebral Microbleeds on Susceptibility Weighted Imaging at 3T and 7T. International Society for Magnetic Resonance in Medicine, Toronto, 2015. Abstract #4393
Figures