2212
Multimodal study of treated brain tumours combining non-Gaussian diffusion MRI and 18F-FET-PET1Institute of Neuroscience and Medicine 4, Research Centre Jülich, Jülich, Germany, 2Department of Neurology, Faculty of Medicine, JARA, RWTH Aachen University, Aachen, Germany, 3Institute of Nuclear Physics, Faculty of Mathematics and Natural Sciences, University of Cologne, Cologne, Germany, 4JARA - BRAIN - Translational Medicine, RWTH Aachen University, Germany
Synopsis
PET using O-(2-18F-fluoroethyl)-L-tyrosine (18F-FET) provides important diagnostic information in brain tumours. In this work, we report a novel combined application of PET imaging and MRI with advanced non-Gaussian diffusion MRI methods including diffusion kurtosis imaging and gamma-distribution function imaging. This is the first multimodal study combining metabolic information from FET-PET and microstructural information gained from non-Gaussian diffusion models. The main goal was to investigate the advantages of such a combined application in tumour assessment and to compare the sensitivity of various non-Gaussian diffusion metrics to the underlying microstructural tissue properties.
Purpose
For the past decade, radiolabelled O-(2-18F-fluoroethyl)-L-tyrosine (18F-FET) has been successfully used in brain tumour diagnosis with positron emission tomography (PET). Due to its high uptake in cerebral gliomas and relatively long half-life time, 18F-FET has become a well-established PET tracer for determination of the tumour extent, recurrence, and treatment monitoring1,2. Hybrid PET-MRI technologies3 allow one to beneficially combine metabolic information gained from PET with standard MRI methods providing high-resolution anatomic visualisation. More recently, advanced diffusion MRI (dMRI) techniques have been increasingly used in the assessment of tumours. In particular, diffusion kurtosis imaging (DKI) has been shown to provide novel biomarkers correlating with tumour grades4,5. However, PET examinations have not been previously combined with DKI or any other non-Gaussian dMRI methods. The aim of this work is to perform a multimodal study of brain tumours combining 18F-FET-PET with non-Gaussian diffusion methods. Among the latter, we included two of such methods, DKI and gamma-distribution function (GDF) imaging. GDF imaging has been recently shown to exhibit high sensitivity to ischemic changes in stroke6 but it has not been yet studied in application to brain tumours.
Materials and Methods
Thirty-eight adult patients with treated high-grade gliomas (HGG) were investigated by 18F-FET PET, conventional MRI, and dMRI after providing written informed consent. MRI experiments were performed using a Hybrid 3T PET-MR Magnetom Trio MR scanner (Siemens Medical System) and included a T1-weighted MPRAGE, contrast-enhanced T1-weighted MPRAGE (T1CE), T2-weighted imaging, T2-weighted FLAIR, and twice-refocused SE-EPI pulse sequence (b-values: 0, 1.0, 2.5 ms µm-2; 30 diffusion gradient directions). 18F-FET-PET1 was performed simultaneously with MR imaging using a BrainPET insert3, 50 min after intravenous injection of 3 MBq of 18F-FET/kg of body weight. Parameter maps from DKI and GDFI were computed as described elsewhere6. The following abbreviations are used below: mean diffusivity (MD), fractional anisotropy (FA), mean kurtosis (MK), tortuosity (TORT), and theta (TT) and kappa (KP) (from GDF). The data from the different modalities were co-registered. Each lesion was segmented into oedema part (based on T2-FLAIR), necrosis (based on T1 and T1CE), and enhancing tumour (based on T1CE). Regions of high metabolic activity were determined based on FET-PET, and were used as reference regions of the metabolically active tumour.Results and Discussion
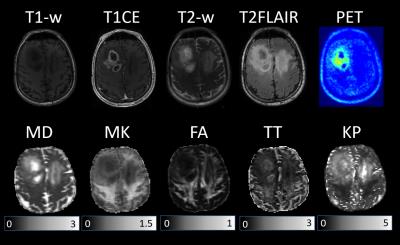
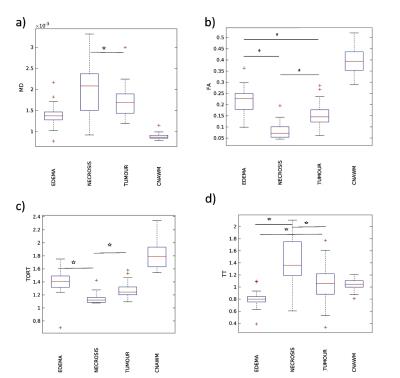
An example of different parameter maps and FET-PET images measured for one tumour patient with treated grade III glioma is shown in Figure 1. The observed lesion exhibits a heterogeneous texture as representative for the most of studied patients. Figure 1 clearly demonstrate complementary contrasts provided by various maps; see, for example, the regions of enhanced activity in FET-PET images, high diffusivity/low kurtosis/lack of enhancement on T1CE within the necrotic regions, enhancing tumour margins in T1CE, MK, and TT, or the region of extensive oedema (crossing the midline) hyperintense in T2 FLAIR, MD and KP but hypointense in MK and TT maps. The contrasts provided by various dMRI metrics were studied separately for each segmented tissue type (solid tumour, necrosis, oedema). Significant differences were observed in MD, FA, TORT, and TT values, see box plots in Figure 2. TT maps showed the biggest relative change between the tissues indicating more clear separation. Sensitivity of non-Gaussian diffusion metrics to different tissue types and the underlying biophysical mechanisms will be discussed.
Conclusions
This is the first study combing anatomic information from conventional MRI and metabolic information from 18F-FET-PET with microstructural information from advanced non-Gaussian dMRI in treated HGG patients. This multimodal approach enabled us to segment observed heterogeneous lesions into 3 tissue subtypes, such as solid tumour, peritumoral oedema, and necrosis, and to perform a separate investigation of their diffusional properties. Significant between-tissue differences were observed for several diffusion parameters. The novel metrics from the GDF imaging allowed one to better distinguish inhomogeneity inside the contrast enhancing tumour and revealed complementary contrasts in comparison to DKI. Taken together, our data demonstrated that our multimodal approach and a novel segmentation can potentially improve the diagnostic assessment of tumour patients and lead to better understanding of biophysical mechanisms governing diffusion in different tissue subtypes.Acknowledgements
No acknowledgement found.References
1. N. Galldiks, K. Langen, R. Holy, et al. Assesment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J Nucl Med 2007;48:519-527.
2. C. Filss, N. Galldiks, G. Stoffels, et al. Comparison of 18F-FET-PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumours. J Nucl Med 2014, 55:540-545.
3. H. Herzog, K. Langen, C. Weirich, et al. High-resolution BrainPET combined with simultaneous MRI. Nuklearmedizin 2011, 50:74-82.
4. S. Van Cauter, J. Veraart, J. Sijbers, et al., Gliomas: Diffusion Kurtosis MR Imaging in Grading, Radiology: volume 263, number 2, May 2012 (radiology.rsna.org).
5. P. Raab, E. Hattingen, K. Franz, et al., Cerebral Gliomas: Diffusional Kurtosis Imaging Analysis of Microstructural Differences, Radiology: volume 254, number 3, March 2010 (radiology.rsna.org).?
6. F. Grinberg, E. Farrher, L. Ciobanu, et al. Non-Gaussian diffusion imaging for enhanced contrast of brain tissue affected by ischemic stroke. PLoS ONE 2014, 9(2):e89225.
Figures