2202
Default Mode Sub-network in Brain Tumor: A Resting State fMRI Study1Department of Medical Imaging, the First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, People's Republic of China, 2Key Laboratory of Biomedical Information Engineering of Education Ministry, Institute of Biomedical Engineering, Xi’an Jiaotong University, Xi'an, People's Republic of China, 3Department of Electronics Engineering, Northwestern Polytechnical University, Xi'an, People's Republic of China, 4Technical University Munich
Synopsis
The aim of the study was to observe the activation of DMN in patients with different types and location brain tumors, and as so to investigate whether the type and location of tumor would affect DMN spatial distribution. In our study, we compared the spatial distribution of DMN both glioma and meningioma. Finally, our findings show that DMN integrity is impaired as a result of tumor, and the DMN spatial distribution could be affected by the location of the brain tumor.
Introduction
Resting state fMRI (Rs-fMRI)
using the blood oxygenation level dependent (BOLD) can reliable and rapidly
detect common functional connectivity network and has sufficient sensitivity
for identifying patterns of network alterations in the brain.1The
default mode network (DMN) is an intrinsic brain system which exists neural
activity and connectivity. Several previous studies had reported that the tumor size
and grade could affects the activation of DMN.2,3 However,
only few studies have attempted to determine the effect of brain lesion
location and type on activity in DMN. The aim of the current study was to
observe the activation of DMN in patients with different types and location
brain tumors, and as so to investigate whether the type and location of tumor
would affect DMN spatial distribution.Materials and Methods
Forty patients with brain glioma (n=20) and meningioma (n=20) were selected (right hands, age range: 27-65 years, mean: 49.27 ± 10.65 years; female: n=21). We employed 3.0 T GE scanner equipped with an eight-channel head coil. Both structural images (3D FSPGR 1x1x1 mm3, 140 slices) and BOLD EPI data (TR/TE = 2500/40 ms, flip angle=90°, 3.75x3.75x3mm3) were acquired. The Rs-fMRI analysis was performed using AFNI and FSL software. The spatial smoothing was performed at 6-mm FWHM Gaussian kernel to reduce noise. The functional connectivity MRI images were filtered with a high pass filter. The functional images were normalized to structural images from the Montreal Neurological Institute (MNI) 152 standard brain space. The ICA analysis was carried out using probabilistic independent component analysis (PICA). The number of components was automatically estimated from the fMRI data by means of a Laplace approximation to the Bayesian evidence of the model order. The resulting maps were thresholded using an alternative hypothesis test based on fitting a Gaussian/Gamma mixture model to the distribution of voxel intensities within spatial maps and a posterior probability threshold of p > 0.5.Results
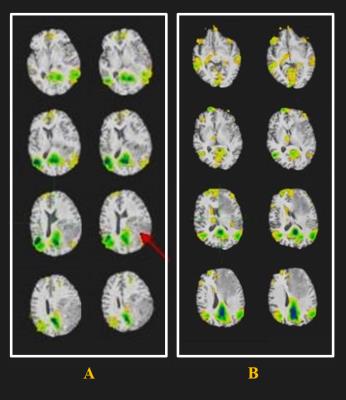
In our study, we compared the spatial distribution of DMN both glioma and meningioma. Our preliminary results showed no significant difference in the activation of DMN between the two groups (Fig.1). Furthermore, the patients were classified into two groups according to the spatial distribution of tumors. Figure 2A shows that the tumor located in the inferior parietal lobe (red arrow), we can observe anterior default mode network in this type. Figure 2B shows that the tumor located in the prefrontal lobe (red arrow), only showing posterior default mode network in this type. Our results suggest that the activation of DMN may be impacted by the location of the brain tumor.Discussion and Conclusions
Our findings show that DMN integrity is impaired as a result of tumor, and the DMN spatial distribution could be affected by the location of the brain tumor. A reduction of DMN functional connectivity between the damaged areas and other functional connected regions could be observed in this study, and the tumor location may have an impact on connectivity of function network. Additional, the tumor location may damage the connection path of DMN. The finding that modifications in DMN spatial distribution were related to tumor location provides additional insight in explaining the relationship brain lesions and cognitive dysfunction, opening new prospects of neurological intervention.Acknowledgements
This research work was funded by National Natural Science Foundation of People's Republic of China (FC No.81171318, No.30870686, No.81000635, No. 61401363) and supported by “the Fundamental Research Funds for the Central Universities”.References
1 Hart M G, Price S J, Suckling J. Functional connectivity networks for preoperative brain mapping in neurosurgery. J Neurosurg.2016;1-10.
2 Ghumman S, Fortin D, Noel-Lamy M, et al. Exploratory study of the effect of brain tumors on the default mode network. J Neurooncol.2016;128(3):437-444.
3 Harris R J, Bookheimer S Y, Cloughesy T F, et al. Altered functional connectivity of the default mode network in diffuse gliomas measured with pseudo-resting state fMRI. J Neurooncol. 2014;116(2):373-379.