2196
Voxel Based Alterations in White Matter Volume following Lipophilic Iron Treatment1Neural and Behavioral Sciences, The Pennsylvania State University - College of Medicine, Hershey, PA, United States, 2Neurosurgery, The Pennsylvania State University - College of Medicine, Hershey, PA, United States, 3Radiology, The Pennsylvania State University - College of Medicine, Hershey, PA, United States
Synopsis
We show that there are observable VBM changes in white matter and grey matter fractions of an aging mouse and these changes are attenuated with a lipophilic high iron diet. This data support the hypothesis that regressive white matter degeneration may be prevented with increased access to CNS iron. Animals that do not have brain iron overloading show longitudinal white matter changes not observed in the iron loaded animals. Furthermore, our data supports the white matter retrogenesis model observed in the aging human brain.
Introduction
The production of myelin is a metabolically intensive process that is imperative in development and persists throughout the aging process. Iron is a transition metal that is predominantly found in oligodendrocytes for the purpose of myelination as it serves as a cofactor for ATP and lipid synthesis1. Iron’s role for early myelination and remyelination in adulthood is crucial and impaired storage and handling of iron can lead to various neuropathologies. Since brain iron levels are predominantly fixed after development, oligodendrocytes may not be able to access iron to facilitate their normal function2. We have shown that the use of a dietary lipophilic iron compound, TMH-Ferrocene (THM-F), can increase brain iron stores. Thus, the purpose of this study was to evaluate the hypothesis that the course of myelination is altered by increasing brain iron stores in adult mice.Methods
To alter mouse brain iron, 10 week old male mice were fed different iron containing diets (n=6/diet): deficient (2-5ppm), sufficient (35ppm), normal (200ppm), 0.1% THM-F (200ppm), and 0.5% THM-F (900ppm). To evaluate the effect of early iron loading, mice fed 0.5% TMH-F were switched to 0.1% TMH-F after three months. All diets were fed ad libitum for 12 months. MRI voxel based morphometry was measured every three months of diet to determine brain fractions. Anesthesia was induced in mice with 3-4% isoflurane and maintained at 1-2% for the duration of the study. All MRIs were acquired using a 7.0T Bruker MedSpec 70/20 system with a 23mm birdcage volume RF-coil. A whole brain anatomical 3D T2-weighted dataset with TR = 350ms, nine echoes (TE=11ms, 11ms spacing), and 100x100x250 μm final resolution was acquired. The SPMmouse package was used to segment the 3D image into GM, WM, and CSF to prepare for DARTEL normalization. Segmented GM and WM tissue classes were imported into DARTEL with SPMmouse before being used to generate a group-specific template image and warps for individual subjects. GM and WM tissue classes were normalized with modulation to create images for use with VBM analysis. Statistical images had a final voxel resolution of 0.2mm isotropic and were smoothed by a 0.4mm isotropic gaussian kernel. Parametric analysis of VBM was performed in SPM8. A 20-region mouse brain atlas was overlaid onto the normalized brains to obtain regional VBM measures. Statistical analysis was performed in SPSS 22 and groups were compared using a Fisher post-hoc test.Results
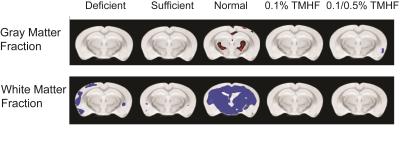
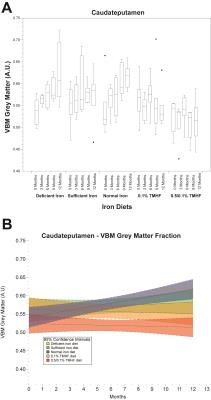
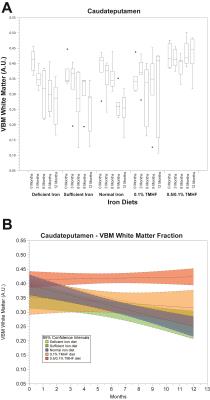
Parametric analysis of deficient, sufficient, and normal iron diets illustrate significant decreases in WM volume over time. These changes are observable in several areas of the brain but are most notable within the normal iron group located around the cerebral peduncles, cortical regions, and corpus collosum (Fig. 1). Iron deficient animals also demonstrate a decrease in cortical WM volume. An apparent increase in GM volume is observed in the normal iron animals. Animals on both ferrocene diets appear to have no significant change over time for both GM and WM fractions. MarsBaR analysis reveals that there are broad trends that are similar among deficient, sufficient, and normal iron diets (Fig. 2A and 3A). These groups significantly change overtime; white matter decreases and grey matter increases. Both ferrocene diets appear to have no volume change for white and gray matter overtime and are significant from the normal iron group at 12 months. 95% Confidence interval trends per dietary group are illustrated in Figure 2B and 3B.Discussion
There are three major findings from this study. First, iron overloading the brain appears to stabilize VBM grey matter and white matter over time. This data supports hypothesis that regressive white matter degeneration may be prevented with increased access to CNS iron3. Second, animals that do not have brain iron overload show longitudinal white matter changes. There is little documentation illustrating the white and grey matter changes in a control mouse and our data supports the white matter retrogenesis model observed in the aging human brain4. Third, this is one of few documentations of the longitudinal usage of VBM in an aging mouse model. VBM in mice has previously been used to observe neuronal insults and/or therapy on the brain5. This reveals that there is much to learn about VBM as a tool to reveal basic aging effects.Conclusion
We have shown that there are observable VBM changes in white matter and grey matter fractions of an aging mouse and these changes are attenuated with a lipophilic high iron diet. Histological investigation of cell body and white matter fibers to better understand what these VBM metrics mean is planned for the future.
Acknowledgements
No acknowledgement found.References
1. Rouault, T. A. & Tong, W.-H. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat. Rev. Mol. Cell Biol. 6, 345–351 (2005).
2. Bartzokis, G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging 25, 5–18 (2004).
3. Connor, J. R. & Menzies, S. L. Relationship of iron to oligodendrocytes and myelination. Glia 17, 83–93 (1996).
4. Brickman, A. M. et al. Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiol. Aging 33, 1699–715 (2012).
5. Sawiak, S., Wood, N., Williams, G., Morton, A. & Carpenter, T. Deformation-based morphometry in the R6/2 Huntington’s disease mouse brain. in Proceedings 17th Scientific Meeting, International Society for Magnetic Resonance in Medicine Honolulu, 544 (2009).
Figures