2194
Dynamic uptake of manganese in the developing mouse brain1Skirball Institute of Biomolecular Medicine, NYU School of Medicine, New York, NY, United States, 2Department of Chemistry, SUNY Plattsburgh, Plattsburgh, NY, United States
Synopsis
Manganese-enhanced MRI (MEMRI) is a powerful technique for imaging rodent neuroanatomy. We investigated differences in the uptake of paramagnetic manganese ions (Mn2+) in the mouse brain at two postnatal days: P7 and P21. We observed higher uptake of Mn2+ in all neuroanatomical regions-of-interest (ROIs) at P7; this increased uptake was reflected in the shorter T1 times at P7 relative to P21. We also observed faster clearance of Mn2+ within the ventricles at P21. Our findings highlight the spatiotemporal differences in Mn2+ uptake and may be useful when planning MEMRI studies in the developing mouse brain.
Introduction
MEMRI is a powerful technique for studying the mouse brain1. The T1-shortening effects of Mn2+ enables the acquisition of high resolution in vivo images of rodent neuroanatomy, with contrast-enhancement. In the mouse brain, MEMRI relies on the uptake of Mn2+ in neural cells, allowing a wide variety of studies into brain anatomical development, activity and axonal connectivity1,2.
Despite the importance and increased use of MEMRI, the dynamics of Mn2+ uptake in the developing mouse brain are still poorly understood. It is critical to characterize the differences in Mn2+ uptake at different neonatal stages in order to better inform study designs, and maximize the contrast enhancement in different brain regions. In this work, we sought to evaluate differences in early Mn2+ uptake at two distinct developmental time-points: P7 and P21.
Methods
Mice were imaged using a Bruker Biospec imaging system with an Avance II console running Paravision 5 software (Bruker BioSpin MRI), interfaced to a 7T 20-cm horizontal bore magnet (Magnex Scientific). A 25-mm (ID) quadrature Litzcage coil (Doty Scientific) was employed for RF transmission and signal detection. Wild-type P7 (n=6) and P21 (n=5) mice were administered MnCl2 (63mg/kg) via intraperitoneal injection before being imaged 30-mins, 150-mins and 240-mins post-MnCl2 injection. For T1 mapping, a separate cohort of P7 (n=3) and P21 (n=7) mice were imaged 6 hours post-MnCl2 injection.
T1-weighted time-course scans. A self-gated 2D gradient echo sequence4 was employed: echo/repetition times, TE/TR=4.7ms/50ms; flip angle=40°; matrix size=384×418 (P7); 384 x 256 (P21); resolution=60mm2; slice thickness=500-mm; number of slices=8; total acquisition time=11 min (P7); 7 min (P21). T1 mapping. T1 maps were acquired using a RARE-VTR (Rapid Acquisition with Relaxation Enhancement–VariableTR) pulse sequence (available on Bruker systems) employing 6 exponentially spaced TRs (from 1065ms to 10000ms). Matrix size=80 x 80 x 80; resolution=240 mm2; 1mm slice thickness; scan time=11 minutes.
Results
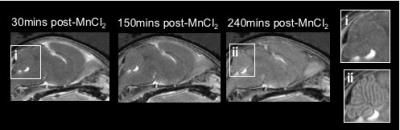
Visual inspection of the P7 imaging data revealed time-dependent enhancement in the brain (Fig.1). Enhancement within the ventricles was readily observable by eye. We also observed striking enhancement within the cerebellum 240mins post-MnCl2 injection, with increasing contrast in the cerebellar folia (Fig.1ii).
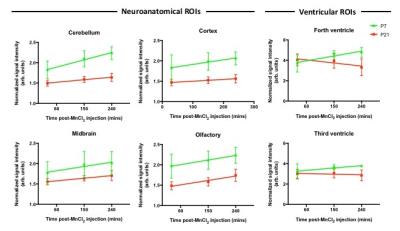
We measured signal intensity (SI) changes in different neuroanatomical ROIs, observing an increase in SI in all ROIs (cerebellum, cortex, midbrain, olfactory bulb) in both P7 and P21 mice (Fig. 2). We also observed an increase in SI within the third and fourth ventricles at P7. Interestingly, we noted decreasing SI within the ventricles of P21 mice from 30 to 240mins.
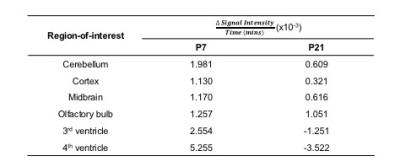
In order to further quantify our observed differences in SI, we used linear regression analysis to estimate the rate of enhancement in each region (Fig.3). We observed that P7 mice exhibited increased uptake rates in all neuroanatomical ROIs relative to P21 mice, while negative uptake rates were observed in the ventricles of P21 mice.
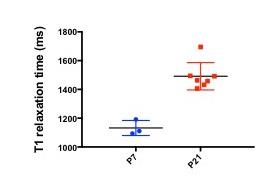
T1 measurements acquired 6 hours post-MnCl2 injection revealed a decrease in mean T1 relaxation time in P7 mice (1132±52.3) relative to P21 mice (1491±94.9) (Fig.4).
Discussion and conclusion
Previous work in the mouse brain has shown that Mn2+ enhancement peaks 24h post-MnCl2 injection5. Our findings of increasing signal intensity in all P7 and P21 ROIs up to 240mins post-MnCl2 injection are in agreement with this prior work. To our knowledge, the increased uptake rates in P7 mice relative to P21 mice have not been previously quantified.
Our findings of negative uptake rates within the ventricles of P21 mice suggest that Mn2+ is being cleared rapidly from these regions. This is in agreement with previous findings in the adult mouse brain5. Conversely, the positive uptake values at P7 indicate that the CSF is still experiencing the T1-shortening effects of Mn2+. It is known that Mn2+ crosses the blood-CSF barrier via the choroid plexus3; the differences in uptake and clearance of Mn2+ at these two distinct postnatal stages may therefore be due to differences in the development and function of the choroid plexus between P7 and P21, which may still be changing at these postnatal stages6.
Our findings of shorter T1 relaxation time in the P7 mice 6 hours post-Mn2+ injection suggest greater uptake of Mn2+ in the younger mice, and are in agreement with our time-course imaging data. Previous work reported a T1 value of 1632ms within the adult mouse cortex at 7T7. Our findings of shorter T1 relaxation time in the cerebellum are expected, and can be attributed to the T1-shortening effects of Mn2+.
In conclusion, our findings highlight the differences in Mn2+ uptake and clearance in P7 and P21 mice. These findings may be of importance to research groups planning MEMRI studies in neonatal mice.
Acknowledgements
No acknowledgement found.References
1. Koretsky AP & Silva AC, NMR in Biomedicine (2004); 17:527–31
2. Szulc, KU et al., Neuroimage (2015); 118:49-62
3. Lee, JH et al., Magnetic Resonance in Medicine (2005); 53(3):640-8
4. Nieman, BJ et al., Magnetic Resonance in Medicine (2009) 61:1148–1157
5. Wadghiri, YZ et al., NMR in Biomedicine (2004); 17:613–619
6. Sturrock et al. Journal of Anatomy (1979); 129(4): 777–793
7. Guilfoyle, DN et al., Magnetic Resonance in Medicine (2003); 49:576–80
Figures