2193
Dynamic Longitudinal DTI Metric Changes of Hippocampus in Developing Non-Human Primate1Keio University School of Medicine, Tokyo, Japan, 2Riken, Wako, Japan, 3Central Institute for Experimental Animals, Kawasaki, Japan
Synopsis
We investigated typical hippocampal development of a non-human primate model, common marmoset, using longitudinal DTI metrics data. Our findings showed dynamical non-linear developmental changes of hippocampal volume, MD and RD especially in its anterior part and suggested its neural turning point would be at puberty. These findings would give insights of disorder and disease of onset specific to developmental period.
Introduction
Hippocampus plays an important role in learning and memory functions. In addition, its morphology and microstructures can be affected by age-related neuropsychiatric disorders and disease such as Autism, and schizophrenia. Thereby, investigating the typical developmental trajectory of this region could serve not only as the key to elucidate the mechanism but also as a normal reference for these disorders or disease.
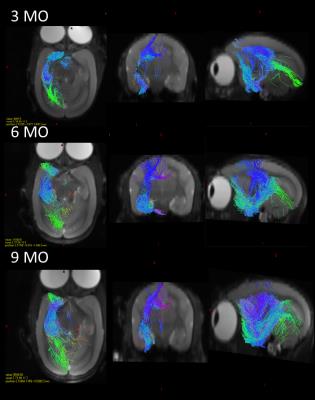
Diffusion Tensor Imaging (DTI) metrics, including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD), have been expected to be valuable indicators of overall cell integrity1. Since such information can be obtained noninvasively by diffusion weighted magnetic resonance imaging method (dMRI), it allows collecting longitudinal data, providing anatomical dynamic changes during brain development. Moreover, recent advancement of dMRI data analysis has promoted us to create virtual in-vivo brain fiber bundle, known as tractography.
Thus, in this study, we collected longitudinal dMRI data of a nonhuman primate model, common marmosets (Callithrix jacchus), to capture the age-related hippocampal microstructural development. We used common marmoset because 1) they reaches adult size and sexually gets matured at around 24 months and 2) like human beings, they are raised and grown by family.
Methods
The 23 animals were obtained from healthy five pairs. They had grown in family, including parents, either/both elder or younger siblings and littermates, at least for first 9 months after birth. We constantly collected their longitudinal T2-weighted and diffusion weighted images (two b0 volumes and 30 volumes with b=1000s/mm2) from 1 to 18 months, total 114 time points, using 7 Tesla Biospec 70/16 MRI scanner (Bruker Biospin GmbH; Ettlingen, Germany) Receiver coil; Bespoke 4ch phased array coil (Takashima Seisakusho Co., Ltd.,Tokyo Japan) with actively shielded gradients at a maximum strength of 700mT/m.
To create the hippocampal region mask and get the sample specific volume, the T2-weighted images were nonlinearly registered to the reference T2-weighted image of marmoset brain, which has its relative hippocampus label map cropped from the published common marmoset brain parcellation map2. Then using the registration information, the hippocampus label was inverted to each brain and modified manually.
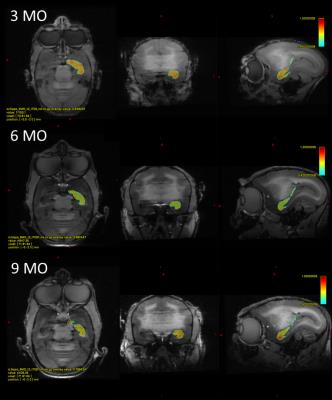
To acquire DTI metrics, we first conducted denoise, eddy correction, and bias field correction, on dMRI data, and then calculated its tensor. Each of the FA, MD, AD, and RD, mean values was statistically analyzed using mixed effect regression analysis, which generally used in longitudinal data, to investigate the age-related changes of hippocampus. Each of the DTI metric heatmaps within Hippocampus was also created to find out especially where the developmental changes were occurred.
Results and Discussion
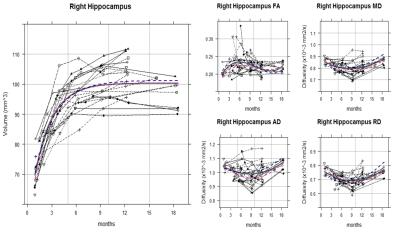
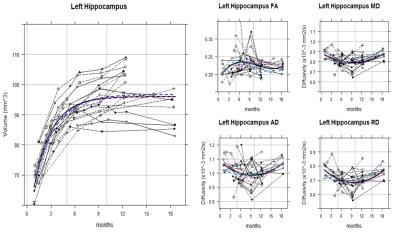
Although the hippocampal volume and DTI metrics (i.e. FA, MD, RD, and AD) values themselves showed great individual differences even among the litter mates, the trajectory shapes of each subject were similar. Indeed, the volume, MD, and RD of hippocampus showed statistically significant nonlinear age-related changes by monthly age. Most of the animals reached the MD and RD developmental turning point at around 9 to 10 months after birth (Figure 1 and 2). For common marmoset, puberty begins at around this period albeit large individual variability. Since DTI metrics can indicated the number and/or size of cells and white matter integrity1,3, increasing MD and RD after 9 months old suggested that pruning of hippocampus started to occur during puberty. That is consistent with molecular and genetic studies4,5 as well and could be a possible explanation of some studies for puberty onset psychiatric disorder such as depression and schizophrenia reported smaller hippocampus volume6.
The RD heatmap and tractography also provided interesting information (Figure 3 and 4): The RD changes occurred more likely on the anterior part of the hippocampus rather than the posterior part. As hippocampal connectivity with other regions are known to differ topographically7, the development within hippocampus would be topographically varies as well. Further studies of investigating the development of the other regions connected with hippocampus such as cingulate, entorhinal cortex, amygdala, prefrontal cortex may help us to elucidate topographic hippocampal anatomy.
Conclusion
We investigated hippocampal development of a primate model, common marmoset, using DTI metrics. Dynamical non-linear changes of hippocampal volume, MD and RD, were observed during development and its turning point was at puberty, suggesting pruning in hippocampus occurred during this period. Moreover, the hippocampal changes were more robust on the anterior part of hippocampus. These findings would be the aid of disorder and disease of onset specific to developmental period.Acknowledgements
This research is partially supported by the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from Japan Agency for Medical Research and development, AMED and Grant-in-Aid for Japan Society for the Promotion of Science(JSPS) Researcg Fellow.References
1. Mori, S., & Zhang, J. (2006). Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron, 51(5), 527-539.
2. Hashikawa, T., Nakatomi, R., & Iriki, A. (2015). Current models of the marmoset brain. Neuroscience research, 93, 116-127.
3. Alexander, A. L., Lee, J. E., Lazar, M., & Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4(3), 316-329.
4. Afroz, S., Parato, J., Shen, H., & Smith, S. S. (2016). Synaptic pruning in the female hippocampus is triggered at puberty by extrasynaptic GABAA receptors on dendritic spines. eLife, 5, e15106.
5. Bakken, T. E., Miller, J. A., Ding, S. L., Sunkin, S. M., Smith, K. A., Ng, L., ... & Shapouri, S. (2016). A comprehensive transcriptional map of primate brain development. Nature, 535(7612), 367-375.
6. Vythilingam, M., Heim, C., Newport, J., Miller, A. H., Anderson, E., Bronen, R., ... & Nemeroff, C. B. (2002). Childhood trauma associated with smaller hippocampal volume in women with major depression. American Journal of Psychiatry, 159(12), 2072-2080.
7. Strange, B. A., Witter, M. P., Lein, E. S., & Moser, E. I. (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience, 15(10), 655-669.
Figures