2185
Phenotyping of transgenic Huntington’s disease minipigs by MR spectroscopyNina Nagelmann1, Frauke Freisfeld2, Robin Schubert 2, Sarah Schramke2, Verena Schuldenzucker 2, Lorena Rieke2, Tamara Matheis2, Harald Kugel1, Ralf Reilmann1,2, and Cornelius Faber1
1Department of Clinical Radiology, University Hospital Muenster, Muenster, Germany, 2George-Huntington-Institute, Muenster, Germany
Synopsis
Huntington’s Disease (HD) is a fatal neurodegenerative disorder, caused by a single genetic mutation, and characterized by deficits in motor coordination, behavior and cognition. A transgenic minipig model of HD has been established. Here, we employ single voxel MRS at 3 T to acquire metabolic profiles from the brain of this model over four years. We show that seven metabolite combinations can be quantified in adolescent and adult minipigs. Pathologic alterations in the phenotype were not observed at the age studied.
Purpose
To assess the feasibility of phenotyping a transgenic minipig model by MRS, and to detect premanifest changes in metabolic profiles of the brainIntroduction
Huntington’s Disease (HD) is an autosomal-dominant neurodegenerative disorder. It is characterized by deficits in motor coordination, behavior and cognition caused by a single misfolded protein (mutant Huntingtin mHTT) originating from a CAG triplet repeat located on the short arm of chromosome 4. Pathological changes have been reported mostly in the cerebral cortex, the caudate nucleus and putamen. Since the discovery of the Huntington gene in 1993, a wide range of transgenic (tg) and knock-in animal models has been established. Here, we investigate minipigs, because this species exhibits a very high genetic homology with humans, has similar body weight (appr. 60-100 kg), and similar body compartments and metabolism. Recently, a transgenic minipig with stable transmission of the HD mutation across several generations was established (Baxa et al., 2013). However, it is still unknown at which age this model develops symptoms of Huntington’s disease. We have used MRS over four years to assess manifestations of alterations in phenotype.Methods
MRS data of female Libechov-minipigs, wild type (wt) and transgenic (tg), aged about 6 months at baseline (M01: nwt=16, ntg=12), at 18 months (M02: nwt=16, ntg=12), at 30 month (M03: nwt=17, ntg=13) and at 42 month (M04: nwt=15, ntg=8 – not closed) were performed on a 3 Tesla Philips Achieva System under general anesthesia with isoflurane. A custom-made minipig array receive coil (RAPID Biomedical GmbH, Rimpar, Germany) was used to obtain high quality in anatomical images and spectroscopy datasets (Fig. 1). To verify a constant quality in MRS data and guarantee correct voxel placement in the striatum, high resolution T1- and T2-weighted volumes were acquired for planning: A T1w 3D gradient echo (TR/TE/FA = 7.5 ms/3.4 ms/9°, FOV = 296x296x225 mm, reconstructed voxel size = 0.51x0.51x0.5 mm, scan time = 32 min) and a T2w 2D turbo spin echo (transversal, TR/TE = 3418 ms/ 80 ms, FOV = 180x180 mm, slices = 30, reconstructed voxel size = 0.19x0.19x2 mm, scan time = 15:22 min) showed best contrast in the basal ganglia. For spectroscopy three single voxels were performed on the minipig brain. The “central voxel” was placed in the median line including striatum bilaterally (measured voxel size: 8x22x8 mm to 8x24x10 mm, depending on brain size) and two smaller voxel (“left” and “right”) were performed on each striatum separately (measured voxel size: 10x6(to 8)x14 mm depending on brain size). Using a standard PRESS-sequence (TR/TE = 2000 ms/32 ms, averages = 176) we acquired MRS data with a scan duration of 6:28 min after shimming (2-5 minutes). MRS data were fitted with the LCModel software, after performing eddy-current correction and water-scaling.Results
The dedicated minipig brain coil allowed to accommodate the animals at all ages and provided images without artefacts, despite the increasing amount of paranasal sinuses in bone with age (Fig. 1). Well resolved spectra were obtained from the central voxel (Fig. 2) with SNR decreasing with age (5.4±1,0 at M01, 5.0±1,3 at M02, 4.8±1,2 at M03, 4.0±0,8 at M04 for the central voxels) (Fig. 3). Water line width increased with age correspondingly (Fig. 3). Spectra from the smaller voxel were too noisy for further analysis (Fig. 3). Seven (combined) metabolites glutamate/glutamine (Glx), creatine (Cre), glycerophosphocholine (GPC), GPC+ phosphocholine (PC), N-acetylaspartate (NAA), NAA+ N-acetylaspartylglutamate (NAAG), myo-inositol (mI) were detected with Cramer-Rao lower bounds ≤ 20. With increasing age, decreasing concentrations of GPC, GPC+PC, and Cre were observed. However, none of these changes differed significantly between wt and tg animals. The other four metabolites showed no pronounced changes with age, nor significant differences between wt and tg animals. For tg animals first alterations in Glx concentration (p= 0.03) were observed between M03 and M04 (Fig. 4). However, for Glx/Cre only a trend was observed (p=0.11).Discussion and Conclusion
Metabolic profiles of the striatum can be obtained at 3 T for adolescent and adult minipigs. In our model of Huntington’s disease no characteristic difference between wt and tg phenotypes was detected in the first four years. In humans the disease has an onset at the age of 40-50 years, which roughly corresponds to 8-12 years in minipigs. The follow-up of this study will show if MRS in addition to MRI volumetry can be established as diagnostic tool for Huntington’s disease, and if our tg minipig model is suitable for interventional and pharmacological trials.Acknowledgements
Funded by the CHDI foundation.References
Baxa M, et al. (2013) A transgenic minipig model of Huntington's Disease. J Huntingtons Dis 2: 47-68.Figures

Fig.1: Left:
Exemplary minipig at M01 prepared for scanning in the custom-made rf coil.
Right: representative T2w images of the minipig brain at M01 and M04, showing
position of the central voxel and increasing sinuses, which cause lower
spectral quality with age.
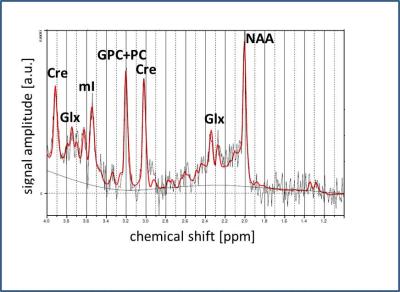
Fig.2: Representative
PRESS spectrum and LCModel fit, obtained from the central voxel of an animal at
M01. Identified metabolites are indicated.
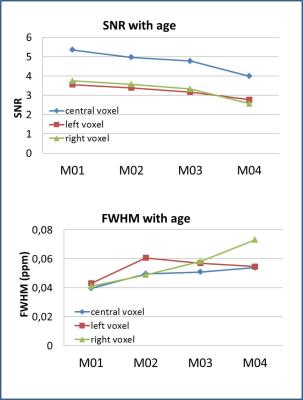
Fig.3: Quality
of the spectra with age. Top: SNR was higher in the central voxel compared to
each unilateral voxel, but decreased with age. Only spectra from central voxel
were used for analysis. Bottom: FWHM of the water line increased with age.
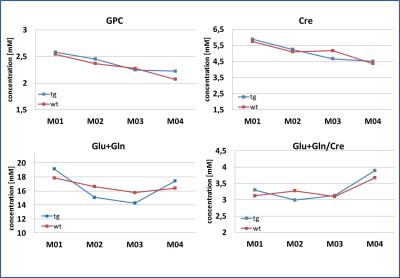
Fig.4: Metabolite
concentrations of Cre, GPC and Glx (= Glu+Gln) decreased with age. No
significant differences were observed between wt and tg animals. For Glx, a
significant increase from M03 to M04 was observed for tg animals only.