Adnan Bibic1,2, Thea Sordia1, Erik Henningsson3, Linda Knutsson1,4, Freddy Ståhlberg2, and Ronnie Wirestam1
1Medical Radiation Physics, Lund University, Lund, Sweden, 2Lund University Bioimaging Centre, Lund University, Lund, Sweden, 3Centre for Mathematical Sciences, Lund University, Lund, Sweden, 4Department of Radiology (Adjunct), Johns Hopkins School of Medicine, Baltimore, MD, United States
Synopsis
In this study, an
improved quantification approach for measuring ASL transit-time parameters is proposed.
The concept is based on multi-TI ASL measurements, where the dynamics of the
inverted spins are described by the Fokker-Planck equation. The random forces
in this equation are assumed to occur due to pseudo-diffusion in the
capillaries and subsequent filtration through the blood brain barrier (BBB). The
obtained time for the intravascular water to distribute from arteries through
the capillary bed and into the parenchyma can, for example, be related to the
capillary function as well as to the integrity of the BBB.
Introduction
Arterial
spin labelling (ASL) for quantification of tissue perfusion employs
magnetically labelled arterial water as an endogenous tracer. Because a
diffusible tracer is used, other relevant perfusion-related parameters, such as
blood mean transit time and blood volume, are unavailable from an ASL study.
Estimation of a tissue impulse response function is further hampered by the
rapid decay of the tracer due to the longitudinal relaxation of arterial blood
water. However, Kelly et al. (1) investigated ASL-based transit-time parameters,
which may serve as useful supplements to CBF information, in an animal study
using a 7T small-bore animal MR system and a continuous ASL sequence. In the
present study, an improved quantification approach for measuring ASL
transit-time parameters is proposed.Method
The presented
methodology is based on a multi-TI ASL measurement, where the dynamics of the
inverted spins are described by a Fokker-Planck equation in one dimension. The
influence of the random forces in the equation is assumed to occur due to
pseudo-diffusion in the capillaries and filtration through the blood brain
barrier, while influence of the drag force occurs only due to the bulk flow in
the arteries with no arterial dispersions. The proposed solution to the Fokker-Planck
equation is based on the bolus tracking solution by Kelly et al. (1), but in the present
implementation a measured arterial input function (AIF) is used as input
function C0 (cf. Eq. 1). The underlying fitting processes were
accomplished by the curve-fitting routine in Mathematica (Wolfram Research
Inc.), using a Nelder-Mead algorithm for unconstrained minimization. The AIF was
interpolated prior to the fitting process, and the integral was solved by
numerical integration.
ASL data were acquired in female Wistar rats (220-250 g body weight) using a 9.4T
MRI animal scanner (Agilent
Inc., Palo Alto, CA, USA). A single-slice flow-sensitive alternating inversion recovery
(FAIR) ASL sequence with a three-shot segmented spin-echo EPI readout was
implemented. For each dataset, the ASL signal at ten different time points was
acquired, starting at inversion time (TI) 300 ms followed by a 300 ms increment
between time points.
The relative
arterial transit time (rATT) and the capillary transit time (CTT) can be extracted by fitting of experimental data to Eq. 1:Results
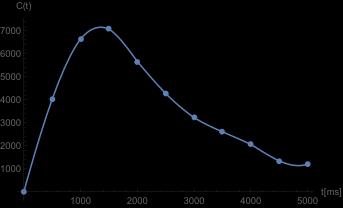
In Figure 1, the measured AIF data points are shown,
with the corresponding interpolation superimposed.
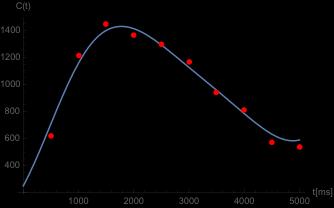
Figure 2 shows experimental c(t) data for the motor cortex ROI in one animal,
together with the resulting model fit. Conclusions
By using the proposed approach, effects of discrepancies in shape between
the assumed and the true AIF are minimized, because the initial distribution becomes
better known when a measured arterial input function is used rather than an
assumed box function.
ASL-based transit-time parameters may provide additional diagnostic
information, not revealed by CBF measurements alone. For example, the time for
the intravascular water to distribute from arteries through the capillary bed
and into the parenchyma can be related to capillary function as well as to the
integrity of the blood brain barrier. Acknowledgements
Lund University Bioimaging Center (LBIC), Lund University, is gratefully acknowledged for providing experimental resources.References
(1) Kelly ME
et al., Phys. Med. Biol. 54 (2009) 1235-1251;