2180
Measuring of Whole Brain Perfusion in the Awake Marmoset Using Continuous Arterial Spin Labeling1Cerebral Microcirculation Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States, 2Biomedical Engineering, Texas A&M University, College Station, TX, United States
Synopsis
The common marmoset, a small New World primate, is a popular non-human primate for transgenic lines of brain disease models and 3D printed helmets to immobilize the head for awake MRI. However, cerebral blood flow (CBF), a crucial component to normal brain functions, has not been measured to date in awake marmosets. In this study, we demonstrate the feasibility of measuring whole brain CBF in the awake marmoset using a continuous arterial spin labeling MRI sequence and dedicated hardware, comprising of a spin-labeling coil and a novel 10-channel phased array in a 7T animal MRI system.
Introduction
The common marmoset, a small New World primate, has become a prime non-human primate model for neuroscience. It serves as an experimental platform for several psychiatric and neurological disorders1, and recently transgenic marmosets have been generated2, 3. Furthermore, marmosets can be acclimatized to the head and body restraint as a mean of immobilization, which is essential for MRI scanning4. Imaging awake marmosets eliminates the use of general anesthesia, avoiding confounds on neurophysiology that hamper the interpretation of experimental results. We have successfully reported the use of awake marmosets in functional and resting state MRI studies5, 6. However, cerebral blood flow (CBF), the key player of functional hyperemia, has not been measured to date in awake marmosets. Also, measuring CBF in awake marmosets is an important way to study the cerebral circulation in both healthy and pathological conditions, as the marmoset’s cephalic arterial pattern is very similar to that of humans7. Hence, the aim of the current study is to demonstrate the feasibility of measuring CBF in the awake marmoset model. We used the continuous arterial spin labeling (CASL) technique with a separate, dedicated labeling coil, which gives the highest theoretical perfusion contrast and no magnetization transfer effects8.Methods
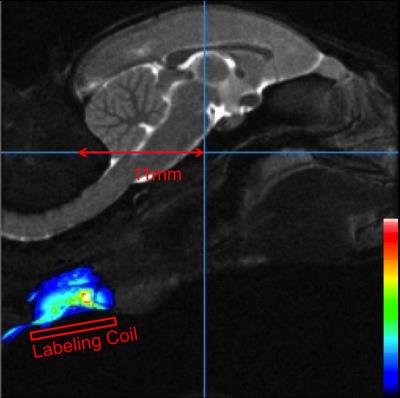
All procedures were approved by the Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke. An adult male marmoset was acclimated to body and head restraint inside a horizontal 7T/30cm MRI spectrometer (Bruker-Biospin Corp., Billerica, USA). The animal was laid in the sphinx position in the cradle, and its head was comfortably immobilized by individualized 3D-printed helmet and chin-piece4. Behavior of the marmoset was constantly monitored by a MR-compatible camera. A custom-built birdcage coil (inner diameter of 10.5cm) was used for transmission and a 10-element phased array RF coil was placed on top of the helmet for signal reception. An active-decoupled rectangular butterfly coil was used to continuously label spins of carotid arteries. The dimension of each rectangular wing was 1cm by 2cm with short edge along the head-foot orientation, as shown in figure 1a. The butterfly coil was attached to the chin piece with an angle of incline around 20 degrees to lay close to the neck when the animal was placed in the sphinx position (figure 1b). The position of the labeling coil could be adjusted along the head-foot orientation and was ca. 11mm posterior to the iso-center for one experiment (figure 2). Bruker’s MAPSHIM routine was used to shim the whole marmoset brain. Perfusion images were acquired using a single-shot spin-echo EPI sequence from eight coronal slices (TE/ TR= 27.6/ 10s; FOV/ thickness= 32×32/ 2mm; matrix= 64×64, Bandwidth= 197KHz). Labeling time was 9673 ms and labeling power was 0.47 W without post-labeling delay. Labeling gradient was 10 mT/m in slice selection direction. For control images, the labeling position was set to 11mm anterior to the iso-center, which was outside of marmoset’s brain. Images were processed by ParaVision or Multi-image Analysis GUI9.Results
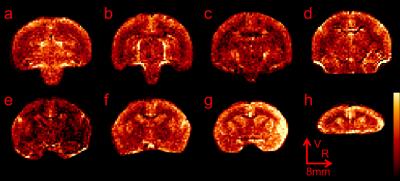
Good spin-echo EPI images were achieved while the marmoset stayed still during the scan. Figure 3 shows an example of baseline images acquired with 16x averaging. Labeling efficiency reached a plateau around 0.47W and the degree of labeling in the center of the brain was calculated to be around 70%. In figure 4, perfusion-weighted images were generated by subtracting control images by CASL images. Excellent contrast between gray and white matter was observed for all slices. The Circle of Willis appeared bright at bottom of the brain (figure 4d, 4e and 4f) due to no post-labeling delay.Discussion
From our preliminary results, we have demonstrated the feasibility of performing CBF measurements in an awake marmoset using CASL with dedicated labeling coil. The degree of labeling of current study was lowered than values reported in similar experiments with anesthetized rats (84% at 4.7T8) and anesthetized macaques (85% at vertical 7T10 and 92% at horizontal 3T11). This is probably due to inefficient flow-driven adiabatic inversion when the carotid arteries posed an angle of incline related to the labeling coil. To minimize the angle of incline, improving of labeling efficiency was underway to optimize the design of labeling coil and its holder. Our MRI setup in awake marmosets may provide a comprehensive platform to study disease models in healthy and transgenic marmosets, especially cerebral vascular disease models.Acknowledgements
We thank Lisa Zhang and Brandon Chen for preparing the animal.References
1. Okano H, Mitra P. Brain-mapping projects using the common marmoset. Neurosci Res 2015; 93: 3-7.
2. Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M et al. Generation of transgenic non-human primates with germline transmission. Nature 2009; 459(7246): 523-7.
3. Park JE, Zhang XF, Choi SH, Okahara J, Sasaki E, Silva AC. Generation of transgenic marmosets expressing genetically encoded calcium indicators. Sci Rep 2016; 6: 34931.
4. Silva AC, Liu JV, Hirano Y, Leoni RF, Merkle H, Mackel JB et al. Longitudinal functional magnetic resonance imaging in animal models. Methods Mol Biol 2011; 711: 281-302.
5. Hung CC, Yen CC, Ciuchta JL, Papoti D, Bock NA, Leopold DA et al. Functional mapping of face-selective regions in the extrastriate visual cortex of the marmoset. J Neurosci 2015; 35(3): 1160-72.
6. Belcher AM, Yen CC, Stepp H, Gu H, Lu H, Yang Y et al. Large-scale brain networks in the awake, truly resting marmoset monkey. J Neurosci 2013; 33(42): 16796-804.
7. Ciochon RL. Evolutionary biology of the New World monkeys and continental drift, Plenum: New York, 1980.
8. Silva AC, Zhang W, Williams DS, Koretsky AP. Multi-slice MRI of rat brain perfusion during amphetamine stimulation using arterial spin labeling. Magn Reson Med 1995; 33(2): 209-14.
9. Lancaster JL, McKay DR, Cykowski MD, Martinez MJ, Tan X, Valaparla S et al. Automated analysis of fundamental features of brain structures. Neuroinformatics 2011; 9(4): 371-80.
10. Zappe AC, Reichold J, Burger C, Weber B, Buck A, Pfeuffer J et al. Quantification of cerebral blood flow in nonhuman primates using arterial spin labeling and a two-compartment model. Magn Reson Imaging 2007; 25(6): 775-83.
11. Duong TQ. Diffusion tensor and perfusion MRI of non-human primates. Methods 2010; 50(3): 125-35.
Figures

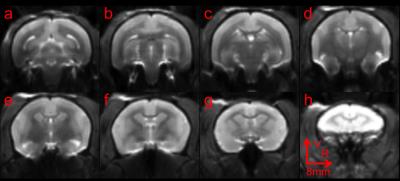
Control spin-echo EPI images of one marmoset. From (a) to (h), the slice position moved from posterior to anterior. Sum-of-squre was used to combined images from the 10-ch phase array. Quadratic Interpolation was used in this figure, but not in further analysis.