2179
Allosteric activation of mGluR4 receptor reversing social behavior deficits modifies the reward-related resting state networks in mu opioid receptor knock-out mice1Medical Physics, University Medical Center Freiburg, Freiburg, Germany, 2Faculty of Biology, Albert-Ludwig-University Freiburg, Freiburg, Germany, 3Department of Psychiatry, Douglas Hospital Research Center, School of Medicine, McGill University, Montreal, QC, Canada, 4Engineering Science, Computer Science, and Imaging Laboratory, Integrative Multimodal Imaging in Healthcare, University of Strasbourg, Strasbourg, France, 5Department of Biophysics and Nuclear Medicine, University Hospital Strasbourg, Strasbourg, France
Synopsis
Mu opioid receptor (MOR) knock-out Oprm1-/- mice exhibit deficits in social behavior and repetitive behavior, which are phenotypes related with autism spectrum disorders (ASD). Thus, MOR deficient mice were recently proposed as monogenic models of ASD. Moreover, a decrease in metabotropic glutamate receptor 4 (mGluR4) levels was found in these mice. A treatment with VU0155041, an allosteric modulator of mGluR4, reversed behavioral deficits in Oprm1-/- mice. Here, we investigated the remodeling on brain connectivity level in Oprm1-/- mice under VU0155041-treatment and found enhancement of positive correlation towards frontal brain areas involved in reward-processing due to the compound.
PURPOSE
The theory of autism spectrum disorders (ASD) being the consequence of abnormal brain development suggests a thorough investigation of brain-wiring in ASD patients1 and the mu opioid receptor knock-out (MOR-KO) Oprm1-/- mice were recently proposed as a monogenic model of ASD.2,3 Investigating the brain connectome of Oprm1-/- mice via resting-state functional magnetic resonance imaging (rsfMRI), strong modifications of reward-aversion circuitry was found.4 Moreover, a down-regulation of the metabotropic glutamate receptor 4 (mGluR4) was reported to accompany the MOR-KO in mice.2 Thus, we investigated here the positive mGluR4-allosteric-modulator VU0155041, which reverses ASD related phenotypes in Oprm1-/- mice2, for its influences on brain connectivity in MOR-deprived mouse brain.METHODS
Animals and treatment: Chronic VU0155041-treatment over 8 days2 was applied to adult male Oprm1-/- mice and respective controls with final treatment administration on the 8th day in the KO-VU group (N=10) as well as in a Ctrl-VU group (with intact MOR gene, N=11), one hour before imaging. Additionally, two groups (Ctrl-NaCl, N=10 and KO-NaCl, N=8) received respective injections of 0.9%-NaCl-solution of matched volume.
MRI experiments: Resting-state fMRI data was collected with 7T small bore animal scanner and mouse head adapted cryocoil (Biospec70/20 and CryoProbe, Bruker, Germany) using single-shot GE-EPI (TE/TR = 10 ms/1700 ms, 200 volumes) with 12 axial slices (0.7mm slice thickness), FOV of 1.92×1.2cm2 and acquisition matrix of 128×80.4,5
Data analysis: rsfMRI data of all animals was assessed via high-dimensional resolution spatial independent component analysis (ICA, 100-ICASSO4,5). Results The resulting patterns were further used as nodes to create the group specific whole brain functional connectivity matrices using partial correlation. Positive and negative correlations were included in subsequent network analysis (graph theory) to identify the “hub” regions – the relay regions of the FC networks (defined as nodes showing over-average normalized strength/diversity). Additional seed-based analysis (p<0.01, detrending/global signal regression) was performed to identify changes in reward circuitry and its core brain areas, in MOR-deprived brain under VU0155041-treatment. The ICA components covering ventral tegmental area (VTA) were used as seeds.
RESULTS and DISCUSSION
Hub-analysis revealed regions known for mGluR4-expression to appear as “hubs” in the resting state network architecture under VU-treatment in both Ctrl and KO-group (see Fig.1). For example, the group of KO-VU specific network hubs comprised all components associated with habenula (Fig.1, comp 71/41), which normally expresses high density of mGluR4, but has downregulated activity in as in MOR-deprived mice.6
To test the hypothesis of remodeling in reward-aversion-circuitry due to VU0155041-treatment in Oprm1-/- animals, seed-based analysis using ICA-components covering VTA (known reward area) was performed (see Fig.2A,B). Ctrl-NaCl group showed strong positive correlations (color-coded by warm colors) towards PAG (arrow 1), SN/MRN (3), HPF (4), habenula (6), striatum (8/11) and ACA (9, Fig.2Ba-f). All these regions are players of reward-aversion-processing known to be disturbed in Oprm1-/- mice.4
VU0155041-administration in Ctrl animals (comparison Ctrl-VU, Fig.2Bg-l with Ctrl-NaCl, Fig.2Ba-f) induced stronger positive correlations from the VTA towards prefrontal cortex (arrow 13), striatum (8), extended amygdala (7) and BST (10). These areas were shown to express high levels of mGluR4,7 thus an effect at these sites supports the idea of VU0155041 strongly influencing brain connectivity in healthy brain.
Upon administration of VU0155041 in Oprm1-/- animals (Fig.2Bs-w), parts of the reward-aversion-circuitry showed similar patterns as those corresponding to Ctrl-VU condition. Amygdala (arrow 7) for instance revealed positive correlation with VTA in accordance with high levels of mGluR4-activity under VU0155041-treatment.
VU-treatment on Oprm1-/- mice (comparison KO-NaCl and KO-VU) induced positive correlations of VTA with amygdala (arrow 7), ACB (12) and PFC (13, Fig.2Bs-w) as compared to negative correlations in the absence of VU-administration (Fig.2Bm-r). In the MOR-deprived brains without VU0155041-injections (KO-NaCl, Fig.2Bm-r) dominance of anti-correlation of VTA with rostral brain areas was detected. Strong anti-correlations towards prefrontal cortex (arrow 13), ACB (11/12) as well as amygdala (7) and lateral hypothalamic area (5) suggest a remodeling of all basal glutamatergic, dopaminergic as well as GABAergic connections forming the main pathways of the reward circuitry.8 A similar rostro-caudal split was reported for the default mode network in adolescents with ASD.9
CONCLUSION
Our study revealed remodeling of reward-aversion-circuitry in MOR-deprived mouse brain under VU0155041-treatment as demonstrated via data-driven hub analysis as well as specific analysis of VTA-connectivity using seed-correlation. The enhancement of positive correlation towards frontal brain areas involved in this circuitry due to compound administration opens a way for potential treatment options in autistic patients with MOR-deficits. More general, this mechanism of acting on a genetically perturbed network via enhancement of alternative pathways might apply for a multitude of pathologies that can be studied using our non-invasive framework applicable for longitudinal studies.
Acknowledgements
This work was supported by the French Academy of Sciences and the NIH (National Institute on Alcohol Abuse and Alcoholism Grant 16658); the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale; Strasbourg University; and grants from the Brain Links Brain Tools cluster of excellence from Freiburg (MouseNet) and European Research Area Network (ERANET-Neuron), AF12-NEUR0008-01-WM2NA.References
1. Mevel, K., Fransson, P.The functional brain connectome of the child and autism spectrum disorders. Acta Paediatr. 2016;105(9):1024-35.
2. Becker, J.A.J., Clesse, D., Spiegelhalter, C., Schwab, Y., Le Merrer, J., Kieffer, B.L. Autistic-like syndrome in mu opioid receptor null mice is relieved by facilitated mGluR4 activity. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2014;39(9):2049–2060.
3. Oddi, D., Crusio, W.E., D’Amato, F.R., Pietropaolo, S. Monogenic mouse models of social dysfunction: implications for autism. Behav. Brain Res. 2013;251, 75–84.
4. Mechling, A.E., Arefin, T., Lee H.-L., Bienert, T., Reisert, M., Ben Hamida, S., Darcq, E., Ehrlich, A., Gaveriaux-Ruff, C., Parent, M.J., Rosa-Neto, P., Hennig, J., von Elverfeldt, D., Kieffer, B.L., Harsan, L.-A. Deletion of the mu opioid receptor gene in mice reshapes the reward-aversion connectome. Proc Natl Acad Sci U S A. 2016 Oct 11;113(41):11603-11608.
5. Mechling, A.E., Hübner, N.S., Lee, H.-L., Hennig, J., von Elverfeldt, D., Harsan, L.-A. Fine-grained mapping of mouse brain functional connectivity with resting-state fMRI. NeuroImage 2014:96, 203–215.
6. Thomsen, C., Hampson, D.R. Contribution of metabotropic glutamate receptor mGluR4 to L-2-[3H]amino-4-phosphonobutyrate binding in mouse brain. J. Neurochem. 1999;72, 835–840.
7. Corti, C., Aldegheri, L., Somogyi, P., Ferraguti, F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience 2002;110, 403–420.
8. Russo, S.J., Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013;14, 609–625.
9. Starck, T., Nikkinen, J., Rahko, J., Remes, J., Hurtig, T., Haapsamo, H., Jussila, K., Kuusikko-Gauffin, S., Mattila, M.-L., Jansson-Verkasalo, E., Pauls, D.L., Ebeling, H., Moilanen, I., Tervonen, O., Kiviniemi, V.J. Resting state fMRI reveals a default mode dissociation between retrosplenial and medial prefrontal subnetworks in ASD despite motion scrubbing. Front. Hum. Neurosci. 2013;7, 802.
Figures

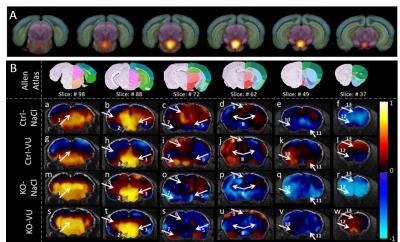
Remodeling of VTA connectivity. A: VTA component overlaid on Allen Mouse Reference Atlas slices (http://mouse.brain-map.org/), used as seed in B: Seed correlation results from VTA component marked by grey arrow 2. Positive correlations represented by warm coloring, negative correlations represented by cold coloring. Atlas slices in the tops row as well as all abbreviations taken from Allen mouse reference atlas (http://mouse.brain-map.org/). 1: PAG, 2: VTA (seed region), 3: MRN/SN, 4: HPF, 5: LHA, 6: LH/MH, 7: AMY, 8: CP, 9: ACA, 10: BST, 11: ACB, 12: ACB (rostral), 13: prefrontal cortex (PFC).