2178
Altered nigrostriatal system in the MPTP Squirrel Monkey model revealed by diffusion MRI at 11.7T.1Center for Neuroimaging Research, Brain and Spine Institute, Paris, France, 2CNRS UMR 7225/INSERM 1127/UPMC UM75, Brain and Spine Institute, Paris, France
Synopsis
Parkinson’s disease (PD) is characterized by neurodegeneration of the dopaminergic neurons in the substantia nigra pars compacta (SNc). The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxin can induce Parkinson syndrome in primate models. We evaluated the nigrostriatal (NS) pathway degeneration in MPTP Squirrel monkeys using diffusion MRI. The results showed significantly increased fractional anisotropy in all NS regions of the MPTP group (SN, caudate and putamen regions). Axial diffusivity also significantly increased in the SN and caudate regions, while radial diffusivities did not show any differences, except a significantly decreased λ3 in the putamen. Those results should help develop preclinical evaluation of PD therapeutics.
PURPOSE
Parkinson’s disease (PD) is characterized by neurodegeneration of the dopaminergic neurons in the substantia nigra pars compacta (SNc). MRI has been used to study neurodegeneration in the nigro-striatal (NS) system in humans and in animal models. Exposure to the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxin can induce Parkinson syndrome in non-human primate models1. Here we sought to evaluate NS pathway injury using diffusion MRI metrics. We hypothesized that MPTP would induce structural alterations of water diffusion in the NS system of treated animals.METHODS
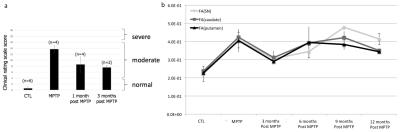
Six adult female squirrel monkeys (saimiri sciureus) were intoxicated with MPTP by repeated intramuscular injections every 3-4 days during 2 months at a cumulative dose of 17.25 to 25.75 mg/kg. Behavioral motor alterations were determined using a clinical rating scale consisting of 5 items including: (1) spatial hypokinesia; (2) body bradykinesia; (3) manual dexterity; (4) feeding; and (5) tremor. Each item had a score of 0-4 resulting in a maximum motor score deficit of 20 points. Brain imaging was performed at the following timepoints: before intoxication, right after the last MPTP injection, 3, 6, 9 and 12 months after intoxication. We used an ultra-high field MRI system at 11.7T (Bruker Biospec 117/16 USR horizontal bore, 750mT/m gradients, Paravision 6.0.1, Ettlingen, Germany) with a custom-built 68-mm diameter transreceive quadrature coil (MRITools GmbH, Germany). All animals were anesthetized with Alfaxalone continuous intravenous infusion. Local first and second order shimming was performed using a Bruker MAPSHIM macro based on a fieldmap acquisition. Anatomical T2-weighted (T2w) images were acquired with a multi-slice multi-echo (MSME) sequence; TR=5000ms; TE=13.5ms; Matrix (Mtx)=256x256; field-of-view (FOV)=5.12x5.12cm2; resolution(res)=200x200μm2; slice thickness=400μm; 128 slices; number of excitations (Nex)=2; acquisition time (Tacq)=42min. For diffusion imaging, a 3D EPI-based sequence was used with TR=300ms; TE=18.5ms; Mtx=160x128x88; FOV=6.4x5.12x3.52cm3; res=400μm isotropic; δ=2.5ms; Δ=8.6ms b-value=1000s/mm2; 80 directions; Tacq=1h17min. A fieldmap acquisition was used to correct EPI geometric distortions. DWI data were preprocessed with standard tools of fsl 5.07: eddy_cor for eddy current correction and fugue for epi distortion correction with the field map acquisition. We then used dtifit to compute the tensor metrics. T2w brain template was created from the 11 individual images. Diffusion images were coregistered to our template by combining linear transformation of diffusion images to the individual T2w and a non-linear transformation of the individual T2w to our T2 template. A total of 11 data points were included in the analysis to compare the MPTP intoxicated condition with the non-intoxicated condition (n=2-3 animals / timepoint). The mean values corresponded to averaged timepoints. Regions of interest (ROI) were manually drawn on our template in the SN, caudate, and putamen. Those regions included both hemispheres. Finally, averaged fractional anisotropy (FA) and eigenvalues (λ1, λ2 and λ3) were calculated within each ROI for each monkey. Group differences between the MPTP and control groups were tested by using non-parametric Kruskall-Wallis rank test and considered significant if p<0.05.RESULTS
FA measurements showed significantly increased FA in all NS regions of the MPTP group compared to the control group (ctrl) (Figure 1a). Axial diffusivity (λ1) also significantly increased in the SN and caudate regions (Figure 1b), while radial diffusivities did not show any differences, except a significantly decreased λ3 in the putamen (not shown). After MPTP injections, the squirrel monkeys display a strong parkinsonian syndrome. While clinical severity increased significantly after the month-period of MPTP exposure relative to the control group (CTL; i.e. before injection), the scores decreased one month after cessation of MPTP injections. After 3 months, diseased animals did not seem to recover further showing behavioral stabilization (Figure 2a). FA and λ1 time series paralleled behavioral scores (Figure 2b) with increased values right after injection then decreased values 3 months later.DISCUSSION AND CONCLUSION
Our results showed significant increases of diffusion metrics in the NS system of MPTP squirrel monkeys suggesting neurodegeneration. Our findings are in accordance with those reported before by Hikishima et al. (2015)2 in MPTP-treated Marmosets. Furthermore, these changes were correlated to the clinical scores of the animals over the duration of 3 months. Overall, this work shows the potential for using diffusion MRI to quantify neurodegeneration in the MPTP squirrel monkey model and should provide tools for preclinical evaluation of PD therapeutics.Acknowledgements
The research leading to these results received funding from the programs “Institut des Neurosciences Translationnelles” ANR-10-IAIHU-06 and “Infrastructure d’Avenir en Biologie Santé” ANR-11-INBS-0006.References
1. Gerlach M and Riederer P. Animal models of Parkinson’s disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm 1996;103(8-9):987–1041. 2. Hikishima K, Ando K, Yano R et al. Parkinson Disease: Diffusion MR Imaging to Detect Nigrostriatal Pathway Loss in a Marmoset Model Treated with 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Radiology 2015;275(2):430-437.Figures