2177
Altered Cerebral Blood Flow and Cerebrovascular Function after Voluntary Exercise in Adult Mice1Mouse Imaging Centre, The Hospital for Sick Children, Toronto, ON, Canada, 2Sunnybrook Research Institute, Toronto, ON, Canada, 3Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
A longitudinal study employing continuous arterial spin labelling MRI with a hypercapnic challenge was used to examine changes in cerebral blood flow with physical exercise in healthy, adult mice. We found that exercise resulted in increases in the normocapnic and hypercapnic blood flow in the hippocampus and that these changes were positively correlated to the volume of the hippocampus following exercise. Interestingly, hypercapnic hippocampal blood flow prior to exercise was predictive of the distance subsequently run and exposure to this voluntary exercise regime was found to reduce these pre-existing blood flow differences.
Purpose
The beneficial effects of physical exercise on brain health are well documented, yet how exercise modulates cerebrovascular function is not well understood. This study used continuous arterial spin labelling (CASL) MRI with a hypercapnic challenge to examine changes in cerebral blood flow (CBF) and vascular function that occur in healthy, adult mice that had undergone four weeks of voluntary exercise.Methods
Male C57BL/6J mice at 12 weeks of age were housed for four weeks in either a control or exercise group. Mice in the exercise group had continuous access to a running wheel with an odometer attached (distance was monitored daily).1 A longitudinal study design was used to track changes in vascular function of individual mice and to determine whether these changes could be attributed to pre-existing differences in blood flow. CBF and vascular reserve were assessed at 12 weeks (week 0) and 16 weeks (week 4) of age using CASL. After the second CASL measurement, a transcardiac perfusion was performed2 and high-resolution ex-vivo anatomical MR images were collected to correlate structural brain differences with the functional measures.
CASL images were acquired at 7 T using a 2D FSE readout (TR=6000ms, ETL=16, TEeff=15ms, slice thickness=2mm, in-plane resolution=250μm, post-label delay=500ms, scan time~5mins).3 Two coronal imaging slices were positioned to transect the motor cortex and hippocampus. A 3-second 9-μT RF labeling pulse (1.3-G/cm gradient) was used to label blood passing through the carotid arteries. The gas mixture inhaled by the mice was cycled between 30% O2/70% N2 and 5% CO2/30% O2/65% N2 for a total of two cycles per slice. Ex-vivo imaging was performed using a 3D FSE pulse sequence (TR=2000ms, ETL=6, TEeff=42ms, resolution=56μm isotropic, scan time~11.5hrs). An automated image registration-based approach was used to assess anatomical differences related to voluntary exercise.1
Results
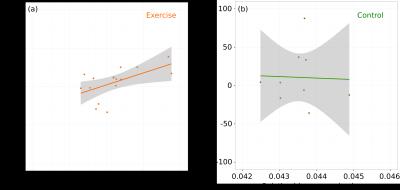
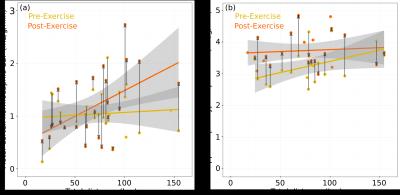
We found that voluntary exercise resulted in increases in the normocapnic and hypercapnic blood flow in the hippocampus; however, there was no overall effect of exercise on the blood flow in the motor cortex (Figure 1). After accounting for the contribution of the normocapnic blood flow, there was a trend towards an increase in the vascular response to hypercapnia in the hippocampus. The change in normocapnic blood flow between week 0 and week 4 was positively correlated to the hippocampal structure volume in the exercise group and not in the control group (Figure 2). Surprisingly, the hypercapnic hippocampal blood flow when measured prior to the start of exercise was predictive of subsequent exercise activity. Moreover, exercise was found to normalize this pre-existing difference in hypercapnic blood flow between mice (Figure 3).Discussion
Using a longitudinal MRI study, we examined the effect of voluntary exercise on vascular function. Whereas we had expected that sustained activity of motor neurons would alter CBF in the motor cortex, we observed no significant changes in blood flow or vascular response to hypercapnia in the motor cortex. This is consistent with our recent work using two-photon fluorescence microscopy that showed exercise did not produce any differences in microvascular network morphology in the motor cortex.4 In contrast, for the hippocampus, four weeks of exercise produced increased basal blood flow and an increased vascular response to a hypercapnic challenge. Here, an increase in both the normocapnic and hypercapnic blood flow in the hippocampus while the brain is at rest after four weeks of exercise suggests the cerebrovascular network has remodeled in response to the increased metabolic demand. Under the assumption that the hypercapnic challenge (5% CO2 for approximately six minutes) is sufficient to maximally dilate the cerebral vessels, exercise increases the maximum blood flow of the hippocampus. The observed change in vascular function supports existing evidence for exercise-induced brain changes in the hippocampus. The positive correlation between the pre-exercise hypercapnic blood flow in the hippocampus and the total distance run suggests there is a pre-existing difference among the mice that predisposes increased exercise performance. In our previous work1 we observed that there is a tendency for exercise to normalize differences in brain structure volumes (i.e. exercise acts to reduce pre-existing neuroanatomical differences) and in light of the current study, this observation can be extended to blood flow. Mice with higher hypercapnic blood flow in the hippocampus were predisposed to exercise but become indistinguishable from their low exercising peers following exercise.Conclusion
In summary, the use of experimental animal models and a longitudinal MRI study design provided an opportunity to examine the relationship between exercise-induced structural changes in the brain and changes in vascular function. This study contributes to our understanding of vascular remodeling following voluntary exercise in healthy adults.Acknowledgements
No acknowledgement found.References
1. Cahill LS, Steadman PE, Jones CE, et al. MRI-detectable changes in mouse brain structure induced by voluntary exercise. Neuroimage 2015;113:175-183.
2. Cahill LS, Laliberté CL Ellegood J, et al. Preparation of fixed mouse brains for MRI. Neuroimage 2012;60:933-939.
3. Cahill LS, Gazdzinski LM, Tsui AK, et al. Functional and anatomical evidence of cerebral tissue hypoxia in young sickle cell anemia mice. J Cereb Blood Flow Metab. 2016; In press. doi:10.1177/0271678X16649194
4. Dorr A, Thomason LAM, Koletar MM, et al. Effects of voluntary exercise on structure and function of cortical microstructure. J Cereb Blood Flow Metab. 2016; In press. doi:10.1177/0271678X16669514
Figures