2175
Behavioral and Image Evidence for Mild Traumatic Brain Injury in Rats with the Skull Helmet1Translational Imaging Research Center, College of Medicine, Taipei Medical University, Taipei, Taiwan, 2Department of Radiology, School of Medicine, Taipei Medical University, Taipei, Taiwan, 3Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan, 4Department of Medical Research, Taipei Medical University Hospital, Taipei, Taiwan, 5Department of Medical Imaging, Taipei Medical University Hospital, Taipei, Taiwan, 6School of Biomedical Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan, 7Department of Biomedica, China Medical University, Taichung, Taiwan
Synopsis
An experimental model of mild traumatic brain injury (mTBI) mimicking the pathological outcomes in mTBI patients found in
Introduction
Mild traumatic brain injury (mTBI), also known as concussive brain injury, is caused by single or repeated force transmitted to the head. In contrast to TBI, which always shows an instantaneous lesion, mTBI does not generate the acute brain pathological change in the early phase.1 Nevertheless, following mTBI, patients may present common post-concussive symptoms (PCS) after months resulting in subsequent neurobehavioral disorders and disables.2 To date, various experimental models of TBI have been widely available to mimic the consequence after brain injury.3 However, a systemic follow-up of both behavioral and imaging outcomes in the clinical-relevant models of mTBI is still limited. In the current study, we modified the impact acceleration injury (IAI)4 model to mimic the pathological outcomes in mTBI patients found in motorcycle accidents in Taiwan. Multi-parametric MRI showed that the gross brain anatomy was well preserved after IAI, while the thinning of the cerebral cortex was found at 50 days. Fractional anisotropy from DTI in the cortex underneath the impact site increased in the acute phase. The severity of injury was controlled by the number and the durations of impact resulting in different behavioral outcomes. The animals after our modified IAI may serve as a clinical-relevant mTBI model to explore early fluid and image biomarkers which are more sensitive to the almost intact brain for early diagnosis of mTBI.Methods
Male Sprague–Dawley rats (250–400 g) were anesthetized with Chloral Hydrate (400 gw/kg) and placed in a stereotactic frame. A circular stainless steel helmet was cemented on top of the skull of the motorsensory cortex (1.5 mm posterior and 2.5 mm lateral to bregma) with dental acrylic to simulate the helmet for motorcycle riders. A weight of 600 g was dropped from a height of 1 m through a stainless-steel tube to the secured impactor aiming to metal helmet. The helmet was removed after IAI. Animals injured with single (n=9) and repetitive IAI within 1 h (n=12) were measured by multi-parametric MRI at pre, 24 h, day 7, 21, 35 and 50. For MRI study, the cocktail of isoflurane and dexmedetomidine was used.5 MRI was performed on a Bruker 7T PharmaScan. T2 map was acquired using multi-spin multi-echo (MSME) sequence with TR = 3 s, effective TE = 18, 36, 54, 72, 90, 108 and 126 ms, FOV= 2.56 × 2.56 cm, matrix size = 192×192, 16 slices, slice thickness of 1 mm and number of averages= 6.6 DTI were acquired using the four-shot spin-echo EPI with TR/TE= 3000/28 ms, FOV= 2.0 × 2.0 cm, matrix size = 96×96, δ/Δ= 5/15 ms, number of b0= 5, number of directions= 30, b-value= 1000 s/mm2 and number of averages= 4. Modified neurological severity score (mNSS) and open field test were performed every 7 days in all animals after IAI and another group of rats with repetitive injury within 3 h (n=6). Regions of interests (ROIs) were placed in the ipsi- and contral-lesional cortex to monitor the DTI matrix change after IAI. Statistical analysis was performed using t-test with significant level set at p<0.05.Results & Discussion
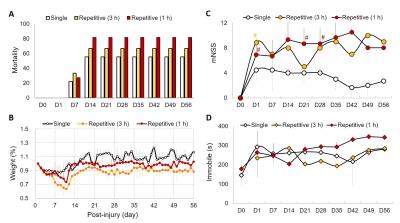
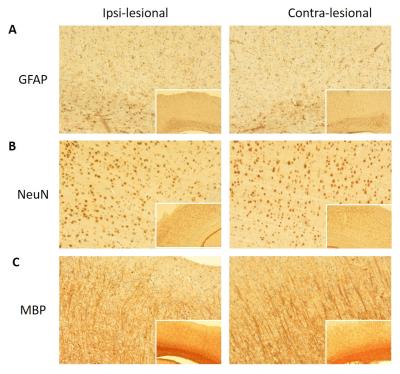
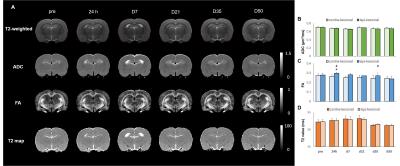
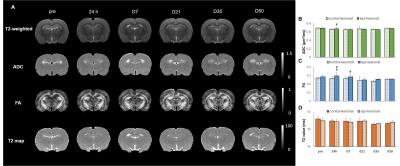
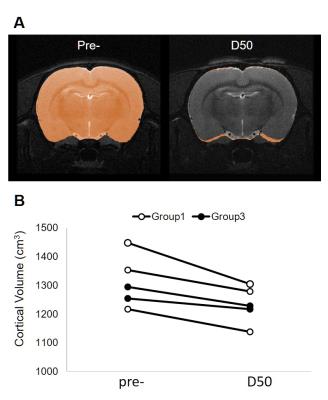
No animal was dead immediately after IAI, while the mortality rate increased after day 7 (Fig 1A), perhaps due to the decrease of body weight within 2 weeks after injury (Fig 1B). At 24 h after IAI, the mNSS dramatically raised because of motor and sensory abnormality, indicating behavioral deficit in animals (Fig. 1C). Recovery of mNSS was found at 42 days after single IAI. During the open-field test, animals after repetitive injury showed the longer period of immobility (Fig. 1D). While significant behavioral deficit was found after IAI, no significant brain contusion, edema, nor structural deformation was found in T2-weigthed images, ADC, FA and T2 maps (Fig 3 & 4). Negative finding in overexpression of astrocytes, loss of neurons or axons at 30 days after IAI was in accordance with our imaging results (Fig 2). At 24 h after IAI, the cortical region underneath the impact site showed significant increased FA value compared to pre-injury, suggesting the impact-induced astrocytic process in the grey matter in the acute phase (Fig 3C & 4C).7 Reduced cortical volume was observed after both single and repetitive IAI (Fig. 5), corresponding to the brain atrophy in mTBI patients in the chronic phase.8 To our knowledge, this is the pilot study showing longitudinal behavioral deficit along with neuroimaging after IAI in rats. Our model maintained the intact brain structure in conventional neuroimaging in the acute phase but induced systemic behavioral deficits may serve as one of the clinical-relevant mTBI models for the following mTBI research.Acknowledgements
This study was funded in part by the Taipei Medical University (TMU103-AE1-B27) and the Ministry of Science andTechnology (MOST 105-2628-B-038-002-MY2 and MOST 104-2923-B-038-003-MY3), Taipei, Taiwan.References
1. Bigler E D, Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc, 2008. 14(1): 1-22.
2. Gosselin N and Tellier M, Patients with traumatic brain injury are at high risk of developing chronic sleep-wake disturbances. J Neurol Neurosurg Psychiatry, 2010. 81(12): 1297.
3. Xiong Y, Mahmood A, and Chopp M, Animal models of traumatic brain injury. Nat Rev Neurosci, 2013. 14(2): 128-42.
4. Fujita M, Oda Y, Wei E P, et al., The combination of either tempol or FK506 with delayed hypothermia: implications for traumatically induced microvascular and axonal protection. J Neurotrauma, 2011. 28(7): 1209-18.
5. Lu H, Zou Q, Gu H, et al., Rat brains also have a default mode network. Proc Natl Acad Sci U S A, 2012. 109(10): 3979-84.
6. Long J A, Watts L T, Chemello J, et al., Multiparametric and longitudinal MRI characterization of mild traumatic brain injury in rats. J Neurotrauma, 2015. 32(8): 598-607.
7. Tu T W, Williams R A, Lescher J D, et al., Radiological-pathological correlation of diffusion tensor and magnetization transfer imaging in a closed head traumatic brain injury model. Ann Neurol, 2016. 79(6): 907-20.
8. Zhou Y, Kierans A, Kenul D, et al., Mild traumatic brain injury: longitudinal regional brain volume changes. Radiology, 2013. 267(3): 880-90.
Figures