2170
Characterization of a Murine High Altitude Exposure Model1Center for Neuroscience and Regenerative Medicine, Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 2Radiology and Radiological Sciences, Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 3Pathology, Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 4Anatomy, Physiology and Genetics, Uniformed Services University of the Health Sciences, Bethesda, MD, United States
Synopsis
MRI is a useful technology for longitudinal assessments, which are critical for understanding vascular changes in the brain. Exposure to high-altitude (HA), hypoxic conditions, e.g. by military personnel, can result in physiological changes and ultimately degradation of neurobehavioral performance. We utilized in vivo MR imaging in mice to examine changes in brain vasculature, myelination and structure and to provide longitudinal insights into pathological changes caused by long-term exposure to HA. We observed changes in T2 that reflect edema/inflammation, fractional anisotropy changes that suggest changes in white matter myelination, and rCBF and VDi imaging indicating adaptations to HA conditions.
Purpose
To utilize MRI for improved, longitudinal characterization of the effects of simulated high altitude exposure in a mouse model, and to correlate the MRI findings with neuropathological and cognitive assessments.Methods
Simulated High Altitude: Male mice approximately 7 weeks of age were ordered from Jackson Laboratories (C57Bl6). Mice were group housed on reverse light cycle. All experiments were approved by the university IACUC. To simulate HA exposure, mice were housed in conventional cages inside a modified hypobaric chamber altered by Reimers System (Lorton, VA) to operate under reduced pressures [1]. Ascent to a simulated altitude of 5000 meters proceeded at 200 meters per minute. Environmental parameters inside the chamber were continuously monitored. Cage maintenance was performed at sea level at least once per week. All mice were monitored daily for signs of distress.
In Vivo MRI: Mice were anesthetized with a mixture of isoflurane and oxygen (30%) & nitrogen (70%) inside a 7T Bruker Biospec 70/20 (Bruker BioSpin Corp. Billerica, MA). All subjects underwent anatomical proton density/T2 weighted (PDW/T2W) imaging, diffusion tensor imaging (DTI) and an arterial spin labeling (pASL)imaging technique for the relative cerebral blood flow (rCBF)calculation[2] , and contrast enhanced Ablavar (Lantheus Medical Imaging, N. Billerica, MA) intraperitoneal injection MR angiography (CE-MRA) to calculate the vasculature density index (VDi). Regions of interests (ROIs) were drawn on T2W images and applied to the co-registered diffusion maps (Figure 1). T2 voxel dimensions: 100 x 100 x 600 μm. DTI (14 directions, b = 1000 s/mm2) @ 200 x 200 x 600 μm. T2 maps and VDi were calculated using VivoQuant (InviCRO, Boston, MA), DTI data were processed and analyzed using TORTOISE [3] software. A cross section study designed allowed for imaging 2-4 mice in 8 separate cohorts at sea level and at high altitude exposure of 1, 3, 6 and 9 month duration.
Neuropathology was
conducted after 12 weeks of simulated HA exposure in the HA mice and in sea level age matched controls to correlate MRI findings with observable changes in
physiology.
Results and Discussion
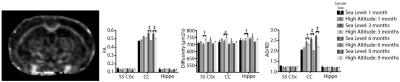
HA exposed mice reveal increased overall T2 values with significant increases in T2 within the corpus callosum (CC) which may reflect ongoing inflammation in this region. The somatosensory cortex (SS Ctx) appears comparatively less affected while T2 values in the hippocampus (Hippo) are slightly elevated following exposure (Figure 2).
Changes in white matter were also found with diffusion tensor imaging as illustrated in Figure 3. In vivo diffusion tensor imaging of control and high altitude exposed C57Bl6 mice revealed a progressive decrease in FA in the corpus callosum with comparatively little change in grey matter regions such as the somatosensory cortex and hippocampus. Mean diffusivity (MD) values also increased, however, both gray and white matter regions were affected. The axial to radial diffusivity ratio (AD/RD) is decreased in exposed mice reflecting increases in RD and decreases in AD in the corpus callosum. All of these observations (low FA, high MD and increased RD) have been correlated with white matter disruption in pathological states such as mild traumatic brain injury. Similar but more subtle changes (elevated MD, AD and RD) in gray matter suggest similar alteration in myelination and/or inflammation. Immunohistochemistry for myelin basic protein in coronal brain sections reveal a deficit of myelination within the neocortex of HA exposed mice.
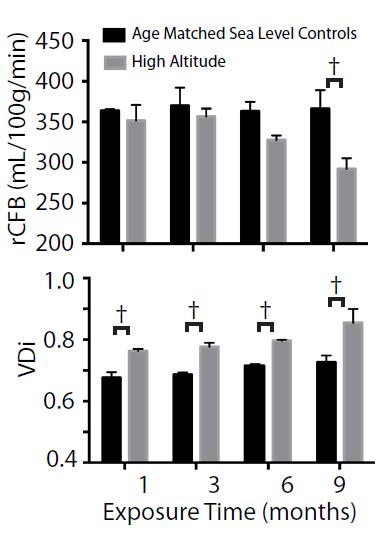
Analysis of pre- and post-contrast enhanced MR angiography revealed a significant increase in the vascular density index (VDi ). pASL imaging revealed a decreased relative cerebral blood flow (rCBF) in the somatosensory cortex (Figure 4). Confirmation with CD31 immunohistochemistry revealed significantly enhanced in vascular density in the neocortex, corpus callosum and hippocampus after 12 weeks at HA.
Conclusions
In vivo MR imaging was able to detect correlates of HA induced pathology in living mice. In particular the vascular density index in the neocortex increased despite lower relative cerebral blood flow, T2 values were elevated in the corpus callosum of exposed mice consistent with inflammation and/or edema,- fractional anisotropy decreased while mean diffusivity increased in the corpus callosum which is also consistent with altered myelination and white matter pathological changes.
In this preclinical study, in vivo MR imaging provided novel
insights into progressive molecular and pathological changes triggered by
exposure to hypobaric hypoxia. It has a future goal of customizing individual
therapeutic interventions for our servicemen and servicewomen who operate in HA
conditions for extended periods of time.
Acknowledgements
Supported by the U.S. Department of Defense in the Center for Neuroscience and Regenerative Medicine (CNRM), with technical support from the CNRM Translational Imaging Facility and Pre-clinical Core, and the Air Force Medical Support Agency.References
1. Cramer, N.P., X. Xu, C. Christensen, et al., Strain variation in the adaptation of C57Bl6 and BALBc mice to chronic hypobaric hypoxia. Physiol Behav, 2015. 143: p. 158-65.
2. Silva, A.C. and S.G. Kim, Pseudo-continuous arterial spin labeling technique for measuring CBF dynamics with high temporal resolution. Magn Reson Med, 1999. 42(3): p. 425-9.
3. Pierpaoli, C., L. Walker, M.O. Irfanoglu, et al. Tortoise: an integrated software package for processing of diffusion MRI data. in International Society of Magnetic Resonance in Medicine. 2010. Stockholm, Sweden.
Figures
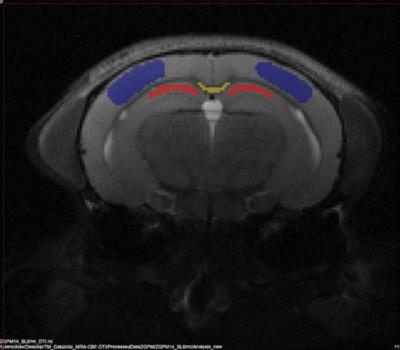
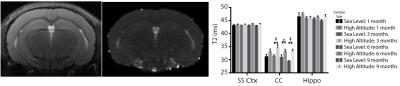
Figure 2. Representative T2W image, corresponding T2 map and changes in T2 for the somatosensory cortex (SS Ctx), corpus callosum (CC), and hippocampus (Hippo). 2-Way ANOVA with Uncorrected Fisher’s LSD post-hoc: * = p < 0.05, ‡ = p < 0.0001