2169
Monitoring glioma heterogeneity during tumor growth using clustering analysis of multiparametric MRI data1U836, Inserm, Grenoble, France, 2Université Grenoble Alpes, Grenoble Institut des Neurosciences, Grenoble, France, 3INRIA, Grenoble, France, 4LJK, Université Grenoble Alpes, Grenoble, France
Synopsis
Brain tumor heterogeneity plays a major role during gliomas growth and for the tumors resistance to therapies. The goal of this study was to demonstrate the ability of clustering analysis applied to multiparametric MRI (mpMRI) data to summarize and quantify intralesional heterogeneity during tumor growth. A mpMRI dataset of rats bearing glioma was acquired during the tumor growth (5 maps, 8 animals and 6 time points). After co-registration of every MR data over time, a clustering analysis was performed using a Gaussian mixture distribution model. Although preliminary, our results show that clustering analysis of mpMRI has a great potential to monitor quantitatively intralesional heterogeneity during the growth of tumors.
Introduction
For tumor diagnosis, histology often remains the reference, but due to tumor heterogeneity, it is widely acknowledged that biopsies are not reliable. There is thus a strong interest in monitoring quantitatively intralesional brain tumor heterogeneity. MRI has demonstrated its ability to quantitatively map structural information like diffusion (ADC) as well as functional characteristics such as the blood volume (BVf), vessel size (VSI), the oxygen saturation of the tissue (StO2), or the blood brain barrier permeability. In a recent study (1), these MR parameters were analyzed independently from each other to demonstrate the great potential of a multiparametric MR (mpMRI) protocol to monitor combined radio- and chemo-therapies. However, to summarize and quantify all the information contained in an mpMRI protocol while preserving information about tumor heterogeneity, new methods to extract information need to be developed. The goal of this study is to demonstrate the ability of clustering analysis (2) applied to longitudinal mpMRI to summarize and quantify intralesional heterogeneity during tumor growth.Methods
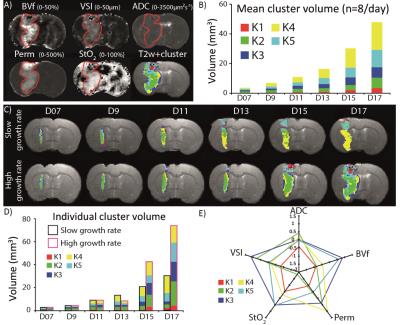
Animal model: The local IRB committee approved all studies. 9L tumors were implanted in 8 rats and imaging was performed every 2 days between day 7 and day 17 post tumor implantation on a 4.7T Bruker system (D7, D9, D11, D13, D15 and D17; respectively). The following mpMRI protocol was acquired at each MR session: a T2-weighted spin echo sequence to obtain structural information over the whole brain, a diffusion weighted EPI sequence to map the Apparent Diffusion Coefficient (ADC) and multiple spin/gradient echo sequences to map T2 and T2*. A Gradient Echo Sampling of the FID and Spin Echo (GESFIDE) sequence was acquired pre- and post-injection of USPIO (133 µmol/kg). A dynamic contrast enhancing sequence was acquired using a RARE sequence (T1w images; n=15, 15.6 sec per image). After the acquisition of 4 images, a bolus of gadolinium-chelate was administered (100µmol/kg). Parametric maps: for each MR session, BVf and VSI maps were computed using the approach described in (3), StO2 using the method described in (4) and the vessel permeability maps (Perm) was calculated as the percentage of enhancement (voxel-wise) within 3 min post injection of gadolinium (cf. fig1-a). Co-registration: each parametric map of each MR session was co-registered to that acquired at the previous time point using rigid registration (SPM toolbox and Matlab). ROI: tumor was manually delineated using the T2w images (Tumor-ROI; Red line in fig1-a). Cluster analysis: parameter values were centered and normalized. Then, a Gaussian mixture distribution (Matlab function called: fitgmdist) was use to performed the clustering analysis of all voxels included in the tumor-ROI. The number of classes inside the mixture was selected by minimizing the Bayesian information criterion (BIC).Results
Firstly, we performed the clustering analysis 9 times using 1 to 9 classes. The optimal classes number, defined by the BIC was 5. Each cluster may be seen as a tissue type, as described Fig.1-E. The result of the clustering analysis is illustrated Fig1-A for one animal. For each of the five clusters (labeled K1 to K5), the evolution of the mean cluster volume over the entire population of tumor is presented Fig 1-B. Note that the sum of the five cluster volumes represents the whole tumor volume. Fig.1-C illustrates the longitudinal evolution of the 5 clusters in 2 animals with different tumor growth rate (slow on the top and high on the bottom). Although the cluster analysis analyzed every voxel independently from each other, one can see that the clustering results are spatially consistent at 1 time point but also over time. Indeed, clusters are spatially grouped: for example, the green cluster is mostly located in the center of the tumor (Fig1-C). Our result shows a difference in cluster composition between the slow and the high growth rate tumors (Fig.1-C,D). For example, in the slow growth rate tumor, the yellow cluster takes more and more space in the tumor overtime (up to 49% at D17) whereas, in the high growth rate tumor, it is the green one. The main difference between the yellow and the green cluster is the strong reduction in StO2 in the green cluster versus the yellow cluster (cf. Fig.1-E).Conclusions
To our knowledge, it is a first study demonstrating the feasibility of performing a clustering analysis on mpMRI data to monitor the evolution of brain tumor heterogeneity in vivo. This approach highlights the type of tissue, which mostly contributes to the development of the tumor. The composition in tissue type could be used to refine the evaluation of chemo and radiotherapies and could contribute to improve tumor prognosis.Acknowledgements
Grenoble MRI facility IRMaGe was partly funded by the French program “Investissement d’Avenir” run by the ‘Agence Nationale pour la Recherche’; Grant 'Infrastructure d’avenir en Biologie Santé' - ANR-11-INBS-0006. BL received a stipend from the "Ligue contre le cancer" and from the "Fondation ARC pour la recherche sur le cancer"References
1. Lemasson B, Bouchet A, Maisin C, Christen T, Le Duc G, Remy C, et al. Multiparametric MRI as an early biomarker of individual therapy effects during concomitant treatment of brain tumours. NMR Biomed. 2015;28(9):1163-73. Epub 2015/08/01.
2. Coquery N, Francois O, Lemasson B, Debacker C, Farion R, Remy C, et al. Microvascular MRI and unsupervised clustering yields histology-resembling images in two rat models of glioma. J Cereb Blood Flow Metab. 2014;34(8):1354-62. Epub 2014/05/23.
3. Tropres I, Grimault S, Vaeth A, Grillon E, Julien C, Payen JF, et al. Vessel size imaging. Magn Reson Med. 2001;45(3):397-408.
4. Christen T, Lemasson B, Pannetier N, Farion R, Segebarth C, Remy C, et al. Evaluation of a quantitative blood oxygenation level-dependent (qBOLD) approach to map local blood oxygen saturation. NMR Biomed. 2011;24(4):393-403. Epub 2010/10/21.
Figures