2163
A Portable Constant-Volume Ventilator for Rodent Hyperpolarized Gas MRI1Biomedical Engineering, Duke University, Durham, NC, United States, 2Center for In Vivo Microscopy, Duke University Medical Center, Durham, NC, United States, 3Medical Physics Graduate Program, Duke University, Durham, NC, United States, 4Radiology, Duke University Medical Center, Durham, NC, United States
Synopsis
Hyperpolarized 129Xe MRI is a powerful probe of lung ventilation, perfusion and gas-exchange. It can be used in both clinical and preclinical settings, where the latter enables rapid discovery of new applications and early testing of therapies in animal models. However, preclinical hyperpolarized gas MRI has been limited to a few expert sites, owing to the challenge of reliably delivering hyperpolarized gas to small animals. Here, we present a constant-volume ventilator that allows for high-resolution hyperpolarized gas imaging and spectroscopy during precisely controlled multi-breath acquisitions. The ventilator is compact, portable, and easy to duplicate and disseminate.
Introduction and Motivation
Hyperpolarized (HP) 129Xe MRI is a powerful method for quantifying lung ventilation, perfusion and gas-exchange in humans1,2. Of particular interest is the wide range of chemical shifts exhibited by 129Xe in biological tissues; these shifts are highly sensitive to physiology and pathology, and can reveal gas-transfer impairment in interstitial diseases like idiopathic pulmonary fibrosis3.
The success of clinical 129Xe MRI has renewed interest in preclinical studies, which offer opportunities to rapidly discover new applications in disease models, test drug treatment response, and validate results with histology. However, preclinical 129Xe MRI is challenging, largely due to the need to precisely deliver HP gas in a way that is MR-compatible and preserves hyperpolarization. Animal lung imaging typically requires data acquisition over multiple breaths. Although HP gas may be delivered via free-breathing4, this tends to use HP gas inefficiently and limit resolution. Obtaining high-resolution 3D images necessitates exact repositioning of the lungs during each breathing cycle, which is most reliably achieved through constant-volume mechanical ventilation. This also enables the tidal volume to be precisely controlled. This is essential for quantitative spectroscopic techniques since xenon chemical shifts are affected by lung inflation5. However, to enable longitudinal studies, these goals must be achieved with non-traumatic peroral intubation.
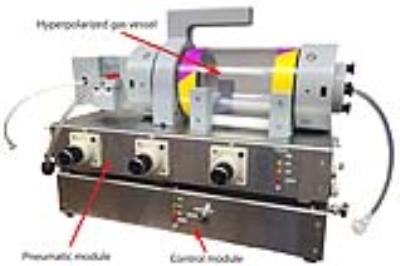
These challenges have limited preclinical HP gas MRI to only a few facilities worldwide. To facilitate more widespread adoption, we present a compact and portable ventilator (Figure 1) that reliably ventilates small animals with HP gases, and demonstrate that it enables high-resolution MRI and accurate spectroscopy.
Ventilator: Key Design Elements and Operation
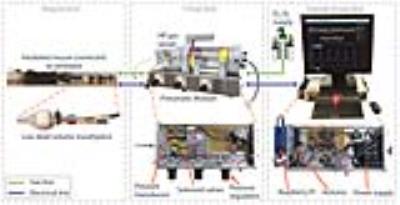
The ventilator uses off-the-shelf components for gas handling, inexpensive electronics, and 3D-printed parts. Figure 1 shows the primary modules and Figure 2 shows the complete set-up. The MR-compatible pneumatic module is housed near the MRI bore, while the control module resides beyond the magnet fringe field. The system user interface runs on a Raspberry Pi single-board computer running Linux, while the timing of gas-delivery valves and scan trigger are controlled by an Arduino microcontroller. Because the Arduino has no operating system and no interrupts, this guarantees uninterrupted ventilation and delivers precise valve timing, which is critical to maximize data acquisition during the short breath-hold (200 ms).
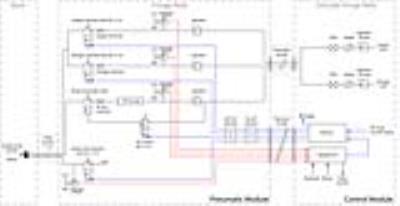
The ventilator delivers a constant tidal volume regardless of pulmonary impedance and eliminates the need for a bulky mixing valve near the mouth of the animal6. Figure 3 shows the schematic of the ventilator. Oxygen and nitrogen flow from supply cylinders to the pneumatic module, wherein regulators and precision high-impedance restrictors with low dead volume, deliver a well-calibrated gas flow rate. The timing and thus volume of gas delivery is controlled by fast-switching solenoid-actuated valves. Oxygen and nitrogen are combined into a single low-dead-volume, 1-meter-long line for delivery to the rodent in the magnet; a second, low-impedance line vents passively-exhaled gases though the exhale valve. When switching from normal ventilation to HP gas delivery, nitrogen is substituted by HP gas, delivered from a perfluoropolymer bag pressurized within a vessel. To preserve polarization, HP gas flows through a polymer-coated pneumatic valve and sapphire orifice restrictor, and through a dedicated line to limit oxygen-induced depolarization. HP gas and oxygen are mixed within a low-dead-volume manifold at the mouse. The manifold is connected to a removable catheter that is used for repeated, non-traumatic peroral intubation.
During the operation of the ventilator, airway pressure is monitored using a transducer coupled to the mixing manifold. The user has full control over the timing of the breathing cycle, the relative position of the scan trigger, and the gas mixture delivered.
Hyperpolarized 129Xe MRI and Spectroscopy
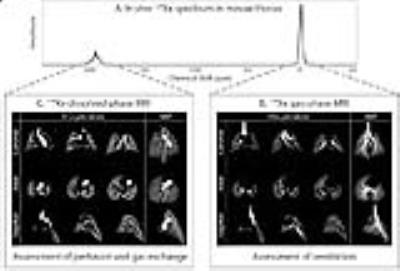
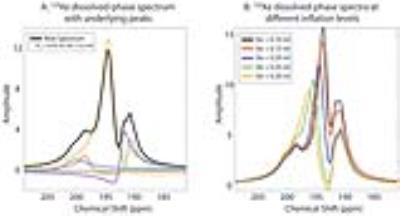
A healthy Balb/c mouse was anesthetized with pentobarbital, perorally intubated, and ventilated with a tidal volume of 0.25 ml, comprising 20% O2 and 80% N2/HP gas. Figure 4 shows 129Xe gas- and dissolved-phase images acquired with 3D radial encoding with 156-µm and 312-µm isotropic resolution, respectively. In the gas-phase 129Xe images, airways down to the 6th order could be visualized. Figure 5A shows a dissolved-phase 129Xe spectrum from the thoracic cavity (TR/TE: 6000/1.2 ms). Its lineshape is strongly influenced by lung inflation (Figure 5B), which makes clear that such studies require a well-defined tidal volume to be consistently delivered.
Dissemination
The compact design enables studies to be conducted at a variety of magnets at our center at Duke University, and now makes dissemination more straightforward. This design was shipped and successfully installed and operated at Oxford University, UK. Moreover, all custom parts are produced by 3D printing, and these designs will be made available for download.
Acknowledgements
NCI R01CA142842, NHLBI R01HL105643, and NIH/NIBIB P41EB015897References
1. Virgincar, R. S. et al. Quantitative analysis of hyperpolarized 129Xe ventilation imaging in healthy volunteers and subjects with chronic obstructive pulmonary disease. NMR in Biomedicine 26, 424-435, doi:10.1002/nbm.2880 (2013).
2. Kaushik, S. S. et al. Single-breath clinical imaging of hyperpolarized 129Xe in the airspaces, barrier, and red blood cells using an interleaved 3D radial 1-point Dixon acquisition. Magnetic Resonance in Medicine 75, 1434-1443, doi:10.1002/mrm.26211 (2016).
3. Kaushik, S. S. et al. Measuring diffusion limitation with a perfusion-limited gas--Hyperpolarized Xe-129 gas-transfer spectroscopy in patients with idiopathic pulmonary fibrosis. Journal of Applied Physiology 117, 577-585, doi:10.1152/japplphysiol.00326.2014 (2014).
4. Iguchi, S. et al. Direct imaging of hyperpolarized 129Xe alveolar gas uptake in a mouse model of emphysema. Magnetic Resonance in Medicine 70, 207-215, doi:10.1002/mrm.24452 (2013).
5. Virgincar, R. S. et al. Establishing an accurate gas phase reference frequency to quantify 129Xe chemical shifts in vivo. Magnetic Resonance in Medicine 00, 00-00, doi:10.1002/mrm.26229 (2016).
6. Nouls, J. et al. A Constant-Volume Ventilator and Gas Recapture System for Hyperpolarized Gas MRI of Mouse and Rat Lungs. Concepts Magn. Reson. Part B 39B, 78-88, doi:10.1002/cmr.b.20192 (2011).
Figures