2161
Determination of RF and acquisition properties for optimal scan performance in 19F-MRI of inhaled perfluoropropane1Newcastle Magnetic Resonance Centre, Newcastle University, Newcastle upon Tyne, United Kingdom, 2Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, United Kingdom
Synopsis
19F-MRI of inhaled perfluoropropane gas allows assessment of lung ventilation properties with a thermally polarised tracer gas. Due to the scarcity of signal from the non-hyperpolarised gas-phase imaging agent, optimal scan protocol design plays a critical role in image quality. Acquisition variables and coil power performance were modelled to determine the optimal image acquisition parameters for a spoiled gradient echo pulse sequence for human 19F birdcage and chest surface coils. Application of optimised scan protocols to human studies shows acceptable image quality for assessment of lung function.
Purpose
MRI offers a safely repeatable modality to evaluate regional pulmonary ventilation, primarily by the use of exogenous imaging agents. Hyperpolarized noble gas MRI has led the field in both clinical research and in introduction to clinical practice, as hyperpolarization enables high signal-to-noise ratio (SNR) images of inhaled tracer gas.1,2 MRI of inhaled perfluoropropane (C3F8, PFP) can exploit the short longitudinal relaxation time of PFP (allowing a high degree of signal averaging3) and therefore offers opportunities for a ventilation imaging method that does not require hyperpolarisation, simplifying the technical requirements for tracer gas MRI. Recently, a number of studies have demonstrated its potential utility as a lung imaging tool.4-6 Due to rapid signal loss from the very short transverse relaxation time of PFP, combined with the low SNR of the thermally polarised gas compared to hyperpolarised gases, the primary challenge to improve capabilities of this technique is to maximise the SNR achieved per unit time. The purpose of our study was to produce an approach for optimisation of the acquisition protocol that accounts for scanner hardware limitations, the in vivo relaxation properties of PFP, and coil RF power performance. This enables identification of the highest possible SNR per unit time attainable for a coil and scanner configuration. We have applied this approach to optimised human studies imaging inhaled PFP.Methods
Modelling: The standard 3D spoiled GRE equation was modified to represent SNR per unit scan time:$$SNR\;per\;unit\;time\propto\sqrt{\frac{NSA}{BW}}\;e^\frac{-TE_{min}}{T_{2}^{\star}}\;\frac{[1-e^\frac{-TR_{min}}{T_{1}}]sin\theta}{[1-e^\frac{-TR_{min}}{T_{1}}cos\theta]}$$
Where NSA is the number of signal averages that can fit within the desired scan length, BW is the acquisition bandwidth, TEmin and TRmin are the minimum achievable echo time and repetition time respectively, and $$$\theta$$$ is the flip angle. The SNR per unit time was calculated for a range of each of these parameters using the in vivo T1 and T2* of PFP.3,4 Coil SAR and power performance properties were implemented in the model, allowing coil-specific relative SNR per unit time to be mapped over a range of input parameters to identify optimal scan acquisition parameters.
Human study: This study was approved by the NHS National Research Ethics Service. Dynamic spoiled gradient echo acquisitions (TE=1.55 ms, TR=4.95 ms, flip angle=50°, FOV=300x300x160 mm3, resolution=10x10x10 mm3, bandwidth=500 Hz/pixel, B1=8 uT) were acquired from 12 healthy volunteers using a Philips 3T Achieva scanner and a 20 cm diameter 19F surface coil (PulseTeq Ltd.) positioned on the volunteers’ upper back. Each subject inhaled a 79% C3F8, 21% oxygen gas mixture with near-vital capacity inspirations for up to one minute. A 2-minute long acquisition with 1.8 s duration dynamics therefore allowed data to be collected on PFP wash-in and wash-out rate. The intra-volunteer reproducibility of measured SNR in the lungs after wash-in was tested using a two-way fixed intraclass correlation.
Results
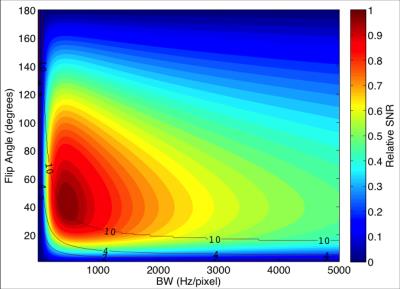
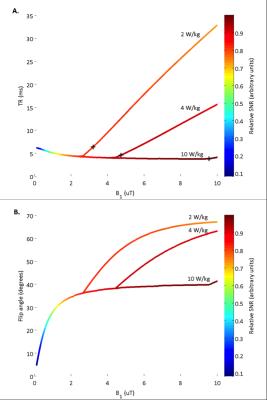
Modelling: The relative SNR per unit time achievable over varying flip angles and acquisition bandwidths for a 20 cm diameter surface coil is shown in Figure 1. Optimal B1 and TR at SAR power depositions of 2, 4 and 10 W/kg are illustrated in Figure 2A to highlight the impact of SAR limits on achievable scan SNR. The corresponding optimal flip angle at each of these B1/TR combinations is shown in Figure 2B. Optimal scan acquisition properties in the human study are dictated by the 10W/kg local SAR limit for this surface coil.
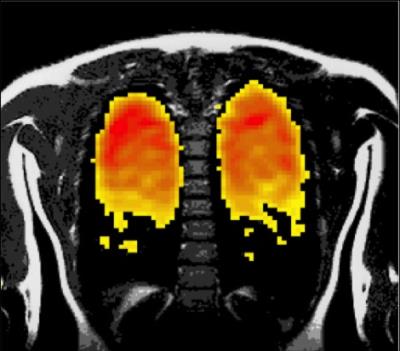
Human study: Near-complete wash-in and wash-out occurred within three breathing cycles with vital capacity inspirations of PFP and room air respectively. An optimised 3D gradient echo scan acquisition protocol resulted in a mean SNR of 13 ± 3 in a 1.8 s duration scan. The intra-volunteer reproducibility of the SNR measurement (ICC(3,1)) was 0.961 (95% CI = 0.863, 0.989). An example 1.8 s 19F image and corresponding 1H anatomical image is shown in Figure 3.
Discussion
The simulations illustrate the impact of scan acquisition parameters on SNR per unit time, and the resulting effect on scan SAR and minimum achievable TR. The information gained from these simulations guided design of scan protocols in the healthy volunteer PFP inhalation study, which showed acceptable image SNR with high temporal resolution. This demonstrates the value of determining the optimal acquisition parameters when imaging a thermally polarised gas. Simulations for a chest volume 19F coil have also been performed, and a human study using this coil is currently underway.Conclusion
Our approach determines optimal scan acquisition parameters for 19F-MRI studies of inhaled PFP, taking into account the in vivo relaxation properties of perfluoropropane and the limits imposed by the scanner and coil hardware.Acknowledgements
This work was funded by a MRC Confidence in Concept grant.References
1. Chang YLV, Quirk JD, Yablonskiy DA. In Vivo Lung Morphometry with Accelerated Hyperpolarized He-3 Diffusion MRI: A Preliminary Study. Magnetic Resonance in Medicine. 2015;73(4):1609-1614.
2. Yablonskiy D, Sukstanskii A, Quirk J. Diffusion lung imaging with hyperpolarized gas MRI. NMR in Biomedicine. 2015.
3. Chang YLV, Conradi MS. Relaxation and diffusion of perfluorocarbon gas mixtures with oxygen for lung MRI. Journal of Magnetic Resonance. 2006;181(2):191-198.
4. Couch MJ, Ball IK, Li T, et al. Pulmonary ultrashort echo time F-19 MR imaging with inhaled fluorinated gas mixtures in healthy volunteers: Feasibility. Radiology. 2013;269(3):903-909.
5. Ouriadov AV, Fox MS, Couch MJ, Li T, Ball IK, Albert MS. In vivo regional ventilation mapping using fluorinated gas MRI with an x-centric FGRE method. Magnetic Resonance in Medicine. 2015;74(2):550-557.
6. Halaweish AF, Moon RE, Foster WM, et al. Perfluoropropane gas as a magnetic resonance lung imaging contrast agent in humans. Chest. 2013;144(4):1300-1310.
Figures