2153
Continuous Cryogen-Free Up-Concentration of Hyperpolarized $$$^{129}$$$Xe Gas1Physikalisch-Technische Bundesanstalt (PTB), Berlin, Germany, 2Helmholtz-Zentrum Geesthacht, Geesthacht, Germany
Synopsis
A semipermeable membrane was utilized to separate the process gas helium from a continuous gas stream containing hyperpolarized $$$^{129}$$$Xe. By this the xenon partial pressure was increased by a factor of ten and the gross amount of helium was removed from the gas stream which was fed from the polarizer to a liquid probe within an NMR spectrometer. Using a sample of dissolved cryptophane-A cage allowed to separate the effects of up-concentration and polarization loss. The increase in xenon partial pressure was still a twofold higher than the polarization loss we have seen and improvements are obvious from the analysis.
Purpose
The potential of hyperpolarized $$$^{129}$$$Xe (hyp-Xe) MR measurements has found an ever increasing interest [1]. However, most of these dissolved phase measurements have not yet been translated to in-vivo. One reason for this might be that animal ventilation with hyp-Xe is an elaborate technique [2,3] requiring hyp-Xe separation utilizing the freeze-thaw method [4,5]. A significant simplification was demonstrated by directly feeding the gas out of the polarizer to a spontaneous breathing animal [6]. This was achieved by omitting the use of helium gas and by working at a total gas pressures below one atmosphere within the polarizer. Thus one had to pressurize the gas mixture afterwards possibly limiting or even losing nuclear polarization at the place of use.
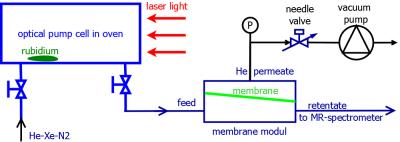
Here, we demonstrate the application of a completely passive system utilizing a semipermeable membrane [7] to separate the gross amount of process gas, namely helium from the hyperpolarized gas stream produced by a hyp-Xe polarizer [8] working at the usual pressure conditions.
Methods
To minimize $$$^{129}$$$Xe polarization loss we enclosed a single flat-sheet membrane [7] within an all perfluoroalkoxy (PFA) module (Fig.$$$\,1$$$). This module was interconnected between the outlet of the hyp-Xe polarizer and the liquid probe saturation setup within a $$$7\,$$$T NMR spectrometer (Fig.$$$\,2$$$).
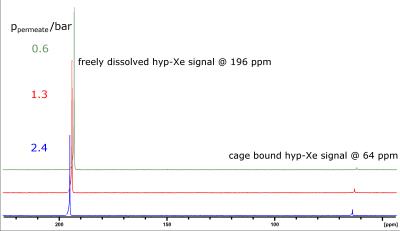
To determine the efficiency of the helium extraction and the potential polarization loss we used a well characterized cryptophane-A cage sample [9,10]. By bubbling the hyp-Xe into a solution of cage concentration $$$[CrA]\approx13\,\mu$$$M one can detect two distinct spectral lines (Fig.$$$\,3$$$). The signal amplitudes of the two lines are given by the following relations:
$$S_{196}=aP_{Xe}sp_{Xe}\qquad\mbox{and}\qquad S_{64}=aP_{Xe}\mbox{[CrA]}\frac{Ksp_{Xe}}{1+ Ksp_{Xe}}$$
where $$$P_{Xe}$$$ is the $$$^{129}$$$Xe nuclear polarization and $$$p_{Xe}$$$ the xenon partial pressure in the retentate from the membrane module fed to the sample. At $$$298\,$$$K the xenon solubility is given by $$$s=0.00432\,$$$M/bar whereas the complex association constant is known to be $$$K=58400\,$$$M$$$^{-1}$$$. The constant $$$a$$$ is a common scaling factor. Thus one can determine from the ratio of the signal intensities $$$S_{196}/S_{64}$$$ the xenon partial pressure to be:
$$p_{Xe}=\frac{\mbox{[CrA]}}{s}\frac{S_{196}}{S_{64}}-\frac{1}{Ks}$$
Results
The increase in dissolved $$$^{129}$$$Xe signal at 196 ppm for a decrease of the permeate pressure is obvious from the NMR spectra (Fig.$$$\,3$$$). This indicates that the dissolved xenon concentration $$$\rho_{Xe}=sp_{Xe}$$$ and thus the xenon partial pressure is increasing due to an increase in helium extraction.
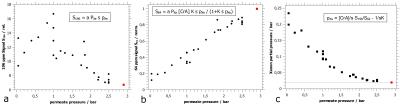
In Fig.$$$\,4$$$a the initial trend (starting from high permeate pressure) of increasing $$$S_{196}$$$ signal is leveling off at lower permeate pressures together with wide variations.
Comparing the $$$S_{64}$$$ signal of the measurement without the membrane module (red point) to measurements with the needle valve closed ($$$\approx2.5\,$$$bar permeate pressure, Fig.$$$\,4$$$b) exhibits that the membrane module introduces a polarization loss of less than 15% when no gas is extracted. The strong decay in the $$$S_{64}$$$ signal due to permeate pressure reduction, however, has to be assigned mainly to $$$^{129}$$$Xe polarization loss of up to a factor of five as the factor $$$Ksp_{Xe}/(1+Ksp_{Xe})$$$ could not change by more than 20% for our parameters.
The deduced xenon partial pressure is plotted in Fig.$$$\,4$$$c showing an increase of up to a factor of ten by reducing the pressure at the permeate port down to vacuum.
Discussion and Conclusion
First of all the results show that we can achieve an considerable up-concentration of xenon gas almost close to the maximum theoretical xenon partial pressure of $$$0.27\,$$$bar in case of complete helium removal. This shows that the membrane in use is perfectly suited for selective helium extraction.
Considering the relatively large surface area the membrane module faces to the gas stream (roughly twice as large as for the used PFA tubing for gas delivery from the polarizer to the NMR sample) the polarization loss solely due to the installation of the module is a remarkably low loss factor. This indicates that the membrane in use is as suitable as PFA – one of the best known polymers to conserve $$$^{129}$$$Xe polarization.
The polarization loss of a factor of five observed for maximum helium extraction can be assigned to the low gas flow when extracting most of the dominant gas mixture. The transmit time for the gas from the membrane module to the NMR-tube increases by at least a factor of four comparing no gas extraction to the maximum helium extraction. However this can be easily compensated by using a $$$2\,$$$mm inner diameter tube instead of the existing $$$4\,$$$mm ID tube again reducing the transmit time by a factor of four. This will be the next tests together with changes in the gas mixture within the optical pumping cell.
Acknowledgements
We thank T. Riemer (Universität Leipzig, Insitut für Medizinische Physik und Biophysik) for help and measurement time at their NMR spectrometer.References
[1] Meersmann, T. and Brunner, E. (ed.). Hyperpolarized Xenon-129 Magnetic Resonance: Concepts, Production, Techniques and Applications; The Royal Society of Chemistry, 2015
[2] Perez De Alejo R, et al. A fully MRI-compatible animal ventilator for special-gas mixing applications. Concepts Magn Reson B. 2005, 26B, 93-103
[3] Nouls J, et al. A constant-volume ventilator and gas recapture system for hyperpolarized gas MRI of mouse and rat lungs. Concepts Magn. Reson. B. 2011, 39, 78-88
[4] Diehuys B, et al. Polarized gas accumulators and heating jackets and associated gas collection and thaw methods and polarized gas products. United States Patent, US7373782, 2008
[5] Hersman W, et al. Large Production System for Hyperpolarized $$$^{129}$$$Xe for Human Lung Imaging Studies. Academic Radiology, 2008, 15, 683 – 692
[6] Hirohiko, I M A I, KIMURA A, FUJIWARA H. Small Animal Imaging with Hyperpolarized $$$^{129}$$$Xe Magnetic Resonance. Anal. Sci., 2014, 30, 157-166
[7] Brinkmann T, et al. Theoretical and Experimental Investigations of Flat Sheet Membrane Module Types for High Capacity Gas Separation Applications. Chem Ing Tech. 2013, 85, 1210-1220
[8] Korchak E S, Kilian W, Mitschang L. Configuration and Performance of a Mobile $$$^{129}$$$Xe Polarizer. Appl Magn Reson. 2013, 44, 65-80
[9] Korchak S, Kilian W and Mitschang L. Degeneracy in cryptophane-xenon complex formation in aqueous solution. Chem Commun. 2015, 51, 1721-1724
[10] Korchak S, Kilian W, Schröder L and Mitschang L. Design and Comparison of Exchange Spectroscopy Approaches to Cryptophane-Xenon Host-Guest Kinetics. J Mag Reson. 2016, 139-145
Figures