2152
Quantifying Changes in Time-Resolved Hyperpolarized 129Xe Spectroscopy among Healthy and IPF Subjects1Center for In Vivo Microscopy, Duke University Medical Center, Durham, NC, United States, 2Medical Physics Graduate Program, Duke University, Durham, NC, United States, 3Department of Biomedical Engineering, Duke University, Durham, NC, United States, 4Department of Electrical and Computer Engineering, Duke University, Durham, United States, 5Division of Pulmonary, Allergy and Critical Care, Department of Medicine, Duke University Medical Center, Durham, NC, United States, 6Department of Radiology, Duke University Medical Center, Durham, NC, United States
Synopsis
The spectral parameters of 129Xe in airspaces, interstitium and red blood cells (RBCs) are sensitive to disease. We sought to test how these parameters change during inhalation, breath-hold, and exhalation, and identify dynamic signatures that distinguish healthy subjects from patients with idiopathic pulmonary fibrosis (IPF). We find in all subjects that the RBC amplitude oscillates at the cardiac pulsation frequency. However, in IPF patients, this oscillation is also prominent in the chemical shift and phase of the RBC resonance. These dynamic metrics are potentially useful biomarkers for disease progression, as well as discriminating between different pathologies that impact gas exchange.
Purpose
129Xe gas, inhaled into human lungs, exhibits distinct NMR resonances corresponding to xenon in the airspaces, interstitial barrier tissues and plasma, and red blood cells (RBCs). While these resonances are of interest for imaging gas exchange, we can also derive sensitive functional metrics from the spectra themselves. For example, six different spectral parameters were found to differ between healthy and idiopathic pulmonary fibrosis (IPF) populations1. Moreover, the temporal dynamics of spectra are also of interest and have been probed by chemical shift saturation recovery (CSSR) techniques to measure gas transfer time constants2. Recently, such methods revealed intriguing cardiac oscillations in the RBC signal amplitude3. Here, we expand on these findings by using steady-state RF pulsing to acquire spectra with higher temporal resolution. Furthermore, we employ time-domain curve fitting tools1 to evaluate the temporal evolution of all spectral parameters (amplitude, chemical shift, linewidth, and phase) for three resonances during a simple breathing maneuver conducted by a cohort of healthy and IPF subjects.Methods
Dynamic spectra were acquired in 14 healthy and 10 IPF subjects, on a 1.5T GE 15M4 EXCITE MRI scanner (GE Healthcare, Waukesha WI). Subjects inhaled a 1-L volume consisting of a 51-mL dose equivalent of hyperpolarized 129Xe4. After inhalation, subjects held their breath for ~8 seconds and then slowly exhaled. During the maneuver, 803 129Xe spectra were obtained over 16 seconds using the following parameters: 512 samples/FID, TE/TR = 0.932/20 ms, BW = 15.63 kHz, flip-angle ≈ 20°. To improve SNR, we employed the spectral improvement by Fourier thresholding (SIFT) method5 using a threshold of twice the noise standard deviation and removed variations exceeding a frequency of 10 Hz. The temporal SNR was further improved by applying a 300 ms boxcar filter prior to decomposing the signal into three resonances using a custom MATLAB toolkit1. For each resonance, the amplitude, chemical shift, linewidth, and phase were reported and plotted as a function of time. To quantify peak-to-peak signal changes oscillating at the cardiac frequency, slower temporal evolution caused by inhalation and magnetization consumption was first de-trended. Assuming sinusoidal behavior, the root-mean-square of the oscillations was calculated $$$x^2_{RMS}=\bar{x}^2+\sigma^2$$$, and converted to a peak-to-peak amplitude $$$A_{pk-pk}=x_{RMS}\times2\sqrt{2}$$$. The peak-to-peak amplitude of the oscillations was compared between groups using a Mann-Whitney U test.Results
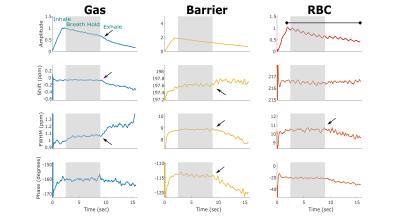
Figure 1 displays the change in 129Xe spectroscopic parameters over time for a representative healthy subject. The breathing maneuver is reflected in three gas phase spectral parameters, showing both inhalation and exhalation effects on amplitude, chemical shift, and linewidth. These effects are also seen, albeit less strikingly, in the barrier and RBC resonances. The chemical shift and linewidth of the two dissolved-phase peaks remains relatively constant, whereas the RBC amplitude exhibits a clear oscillation at the heart rate.
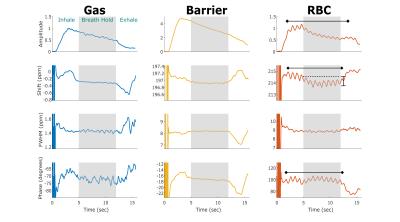
Figure 2 displays the same dynamic spectroscopic information for a subject with IPF. Again, the gas-phase parameters reflect the inhale and exhale dynamics, along with a clear increase in the chemical shift and decrease in the linewidth of the barrier resonance upon exhale. Similar to the healthy subject, the RBC amplitude oscillates at the heart rate, but its chemical shift starts ~1.5 ppm lower than in the healthy volunteer and then decreases by ~0.5 ppm during the breath-hold. The most prominent difference from the healthy subject is that cardiac oscillations are also evident in the RBC chemical shift (~0.4 ppm peak-to-peak amplitude) and phase (~6.2°).
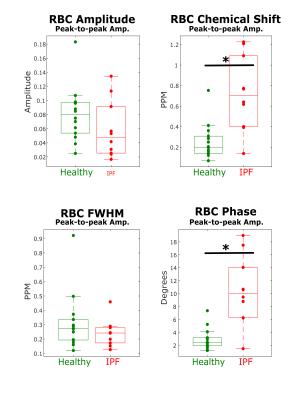
The peak-to-peak changes in each of the RBC spectral parameters are displayed in Figure 3. The amplitude of the cardiac-associated oscillations in chemical shift and phase were significantly different between IPF and healthy subjects (shift, p=0.0026; phase, p=0.0012).
Discussion
It is striking to find that nearly all spectral parameters reflect dynamics associated with the breathing maneuver. However, the RBC spectral dynamics are particularly informative. In the healthy volunteer, the RBC chemical shift remains fixed during the breath-hold likely reflecting that healthy lung has enough reserve capacity to maintain a well-oxygenated environment. The IPF subject starts with a lower RBC shift, which continues to decrease throughout the breath-hold. This may indicate that capillary blood in IPF lungs starts more poorly oxygenated and is unable to maintain that level of oxygenation throughout the breath-hold.
The cardiac oscillations seen in the RBC chemical shift and phase of IPF subjects were particularly striking and may suggest that this method is sensitive to changes in blood oxygenation on the timescale of the cardiac cycle. Since this fluctuation is larger in IPF subjects, it suggests this metric has the potential to be used as a marker of disease or to measure disease progression.
Acknowledgements
R01HL126771, R01HL105643, P41 EB015897, Gilead SciencesReferences
1. Robertson SH, Virgincar RS, Bier EA, He M, Schrank GM, Smigla RM, Rackley C, McAdams HP, Driehuys B. Uncovering a third dissolved-phase 129Xe resonance in the human lung: quantifying spectroscopic features in healthy subject and patients with idiopathic pulmonary fibrosis. Magn Reson Med 2016;doi:10.1002/mrm.26533.
2. Chang YV, Quirk JD, Ruset IC, et al Quantification of Human Lung Structure and Physiology Using Hyperpolarized Xe-129. Magn Reson Med 2014;71(1):339-344.
3. Norquay G, Leung G, Stewart NJ, Wolber J, Wild JM. 129Xe chemical shift in human blood and pulmonary blood oxygenation measurement in humans using hyperpolarized 129Xe NMR. Magn Reson Med 2016;doi:10.1002/mrm.26225.
4. He M, Robertson SH, Kaushik SS, Freeman MS, Virgincar RS, Davies J, Stiles J, Foster WM, McAdams HP, Driehuys B. Dose and pulse sequence considerations for hyperpolarized (129)Xe ventilation MRI. Magn Reson Imaging 2015;33(7):877-885.
5. Doyle M, Chapman BLW, Balschi JA, Pohost GM. SIFT, a postprocessing method that increases the signal-to-noise ratio of spectra which vary in time. J Magn Reson B 1994;103:128–133.
Figures