2149
Implications of B0 and B1 inhomogeneity for bSSFP imaging of hyperpolarized media1University of Sheffield, Sheffield, United Kingdom
Synopsis
The effect of B0 and B1 transmit inhomogeneity on 3D bSSFP lung imaging with hyperpolarized 129Xe was simulated using flip angle and off-resonance frequency maps in combination with the matrix product operator approach to predict 129Xe magnetization dynamics and associated bSSFP signal distributions. B1-related signal drop-off was predicted in posterior and some anterior regions, whilst central regions were generally robust to flip angle variations. Regions of high off-resonance frequency near the diaphragm resulted in low simulated bSSFP signal, corresponding spatially to banding artifact locations. When combined, the two factors led to mean bSSFP image intensity variations ~15-20%.
Purpose
Balanced steady-state free precession (bSSFP) sequences allow high SNR imaging of lung ventilation with inhaled hyperpolarized 129Xe (HP129Xe), 1-3 and show promise for body HP13C applications. 4 However, bSSFP sequences characteristically suffer from banding artifacts related to off-resonance (Δf0), which is prominent in the thorax in regions of high susceptibility difference. Furthermore, flexible transmit-receive (T-R) RF coils with imperfect B1 homogeneity are routinely used for HP129Xe lung imaging, as they are low cost and afford patient comfort and high filling factors. However, the sensitivity of HP129Xe 3D bSSFP to B0 and B1 inhomogeneity has not been well characterized. In this work, a technique for simulating the distribution of HP129Xe 3D bSSFP signal from flip angle (α) and Δf0 maps is presented and experimentally validated.Methods
3D Δf0 and α mapping was performed in two healthy volunteers (male; 26 (V1) and 34 (V2) years) at 1.5T with a close-coupled, dual-Helmholtz, flexible T-R RF coil. Subjects inhaled ~800mL HP129Xe mixed with ~200mL nitrogen and maintained breath-hold for ~15s whilst Δf0 and α maps were acquired sequentially. Δf0 maps were obtained using an interleaved 3D SPGR sequence with 2 echo times: TE1/TR1=1.4/3.2ms; TE2/TR2=4.6/6.4ms; field-of-view (FOV), 40cm; 16 coronal slices (no gap); in-plane matrix, 32x32; slice thickness, 16mm; α, 2°; bandwidth, ±8kHz; scan time, 5s. α maps were obtained using 2 back-to-back 3D SPGR acquisitions: parameters as above except input α, 2.5°; TE/TR=TE1/TR1. In one volunteer (V2), Δf0 and α maps were also obtained in separate breath-holds such that higher flip angles (3.5° and 4°, respectively) could be used to induce greater RF depolarization and improve SNR to validate the same breath-hold procedure. From the resulting ∆f0 and α maps, the HP129Xe 3D bSSFP signal was modeled by extending the matrix product operator approach in 2,3 on a regional basis to derive simulated bSSFP signal maps.Results and Discussion
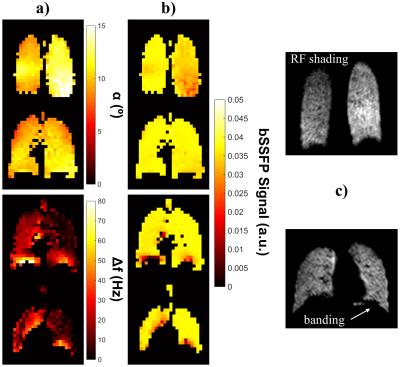
The flip angle of the coil was homogeneously distributed across the central regions of the lungs, whilst some left-right inhomogeneity and increased α values were measured in posterior regions. The global mean variation in α was 24%, 25% and 16%, for V1, V2 (same and separate-breath experiments), respectively. The separate-breath experiment (Fig.1) isolated the influence of α inhomogeneity on bSSFP signal distribution and showed that regions of high α were associated with signal drop-off, whilst regions with little α variation exhibited uniform signal intensity. In previous work, 3 we reported that 129Xe 3D bSSFP SNR is robust to global variations in α ~1-2° (≲20% of the optimum flip angle, ~10°). An example 129Xe bSSFP lung image depicting left-right α-related “shading” of signal intensity is shown in Fig.1c.
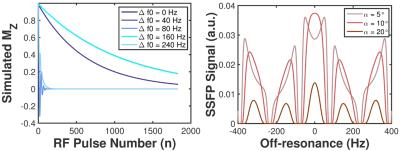
Fig.2a illustrates the global effects of off-resonance on the longitudinal magnetization of HP129Xe. Increasing the degree of off-resonance results in more rapid signal decay and elongation of the oscillatory behavior of the longitudinal magnetization. This corresponds to periodic nulling of bSSFP signal as a function of off-resonance frequency (Fig.2b). ∆f0 maps showed homogeneously distributed off-resonance frequencies across the lungs in the anterior-posterior direction, however in some slices, high Δf0 values (≲80Hz) were observed in the basal regions (near the diaphragm), as illustrated in Fig.1 and Fig.3a. The global mean ± standard deviation of Δf0 was 13.7±10.1Hz, 12.4±9.1Hz and 15.2±13.3Hz for V1, V2 (same and separate-breath experiments), respectively. Resulting 129Xe 3D bSSFP signal was generally uniformly distributed across the lungs, with some significant regions of low intensity near the diaphragm, corresponding spatially to high Δf0 regions. Moreover, these regions qualitatively matched those of banding artifacts occasionally detected in HP129Xe bSSFP imaging (Fig.1c and 1,3).
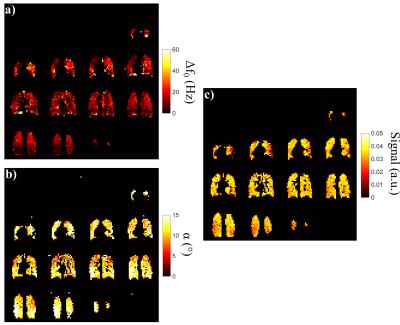
An example complete same-breath dataset (∆f0, α and signal maps) is shown in Fig.3. Whilst separate-breath results clearly distinguished the signal distribution characteristics associated with the inhomogeneity of the two parameters, the combination of these effects in the same-breath experiments resulted in a patchier signal distribution. The combined α and Δf0 variability resulted in 15.6%, 20.0% deviation in mean bSSFP signal across the lungs for V1, V2 respectively (mean ± standard deviation of signal: 3.2±0.64 and 3.3±0.52, respectively).
Conclusion
A method for assessing intensity variations in HP129Xe 3D bSSFP lung images by simulating the signal dependence on α and Δf0 distributions across the thorax has been presented. This technique should improve the distinction of banding artifacts, or transmit B1-related signal loss, and true ventilation abnormalities in HP129Xe images, and may permit “B0 and B1 bias correction” of bSSFP images in future. This work is readily applicable to HP13C bSSFP studies which use analogous coils at a similar Larmor frequency to 129Xe.Acknowledgements
No acknowledgement found.References
1: J. P. Mugler III, T. A. Altes, I. C. Ruset, et al. Hyperpolarized Xe-129 Ventilation Imaging using an Optimized 3D Steady-state Free-precession Pulse Sequence. Proc. ISMRM, Hawai'i. 2009;#2210.
2004;61(7):1025-1029.
2: J. M. Wild, K. Teh, N. Woodhouse, et al. Steady-state free precession with hyperpolarized 3He: experiments and theory. J Magn Reson, 2006;183:13-24.
3: N. J. Stewart, G. Norquay, P. D. Griffiths, J. M. Wild. Feasibility of human lung ventilation imaging using highly polarized naturally abundant xenon and optimized three-dimensional steady-state free precession. Magn Reson Med. 2015;74(2):346-352.
4: E. S. S. Hansen, N. J. Stewart, J. M. Wild, et al. Hyperpolarized 13C,15N2-Urea MRI for assessment of the urea gradient in the porcine kidney. Magn Reson Med, 2016;doi:10.1002/mrm.26483.
Figures