2146
Optimization of free breathing radial DCE-MRI protocol for quantitative clinical evaluation of pleural malignancies1Radiology, Brigham and Women's Hospital, Boston, MA, United States, 2Siemens Healthcare USA
Synopsis
Clinical diagnosis of pleural malignancies and evaluation of their treatment response to novel anti-angiogenic agents would benefit from quantitative clinical DCE-MRI. Implementation of robust DCE-MRI of the thorax is challenging given the presence of significant respiratory motion. We optimized a clinical DCE-MRI protocol based upon the Radial Stack of Stars acquisition scheme to obtain 3-dimensional motion insensitive DCE-MRI of pleural malignancies. When compared to other commonly used DCE-MRI protocols with cartisian acquisitions, our free breathing protocol demonstrated good SNR, minimal motion down to a spatial resolution of 2mm3.
PURPOSE:
To optimize a free breathing clinical protocol for quantitative DCE-MRI of pleural malignancies.METHODS:
The study was approved by our institutional IRB. We implemented free-breathing gradient echo DCE-MRI protocols using a Radial Stack of Stars sequence (RADIAL-VIBE)1 time resolved with a Golden angle scheme, a Cartesian acquisition (FLASH) and Time-resolved Angiography With Interleaved Stochastic Trajectories (TWIST) sequence [TR = 2.5ms, TE = 1ms, FA = 10∘, 3s time resolution, 2 x 2 x 2mm3 spatial resolution, coronal slicing covering the entire thorax; 0.1mmol/kg of Magnevist was injected 30s into each dynamic scan]. Subjects with known pleural-based tumors (malignant mesothelioma, N = 15, or abutting the pleura in the lung, N = 7, age 42-81, males = 14, females = 8) were imaged with one of the above sequences. The SNR, CNR and 3D displacement of the pleural margins for each sequence were compared. Motion of the thorax along orthogonal directions across over time were assessed by analyzing the corresponding 2D spatial temporal image (2DST, Figure 1) 2-3. 2DST images were obtained from stacked time image sequences for each slice of the three-dimensional dataset. 2DST images chosen at the plane of the left and right pulmonary apices were used to evaluate superior-inferior and anterior-posterior motion. 2DST images at the plane at the level of the middle mediastinum were used to evaluate left-right motion. A Canny line detection algorithm, supplemented by a gradient filter, was used to segment the line profiles. All analyses were performed with MATLAB. SNR and CNR were compared using one-way ANOVA analysis. The 95% confidence intervals for the mean displacement was calculated for each line profile.RESULTS:
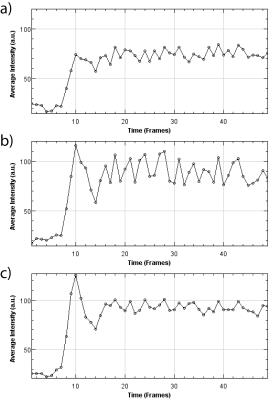
RADIAL VIBE, FLASH and TWIST sequences did not demonstrate significantly different SNR and CNR (Figure 2). Line profile analysis showed minimal motion in the anterior-posterior and lateral directions. Maximal displacement was noted in the superior-inferior direction (mean mm ± 95% confidence interval, Lung apices RADIAL VIBE: 0.24± 0.39, TWIST: 2.02±1.72, FLASH: 0.61±0.67; Diaphragm RADIAL VIBE: 0.30±0.45, FLASH: 6.34±3.36, TWIST: 4.6±2.02, Figure 3). This was consistent with significant distortion seen in the lesion signal intensity time curves for FLASH and TWIST sequences (Figure 4).DISCUSSION:
Novel anti-angiogenic 4 and immunotherapies 5 are being used to treat pleural malignancies. Imaging biomarkers that can evaluate tumor vascularity and vessel permeability will greatly aid their diagnosis and treatment. DCE-MRI of the thorax is challenging given the presence of significant respiratory motion. Several approaches have been developed to address this issue. However, approaches to date can be limiting in terms of temporal resolution and spatial coverage. Moreover, many of the approaches were developed for lung masses, harnessing the high contrast between the lesion and lung parenchyma as part of the data coregistration algorithm 6. This may not always be possible for pleural lesions. Thus, it is important to optimize a DCE-MRI protocol that is applicable for imaging pleural malignancies. Moreover, given the clinical status of these patients the ideal protocol needs to be free breathing compatible and simply implemented. Few studies to date have explored the use of DCE-MRI to characterize pleural-based lesions 7-9. Given the challenges noted above, we optimized our RADIAL-VIBE sequence to minimize reconstructed image co-registration, while keeping the protocol free-breathing. Our optimized radial DCE-MRI sequence provided excellent motion robustness. On average, there was minimal (sub-voxel) displacement of the pleural border in three dimensions using the radial sequence, compared to FLASH and TWIST. This is consistent with the fact that the radial sequence over samples only the center of k-space, thereby reducing motion artifacts 10. TWIST was the least robust to motion. While it also preferentially samples the center of k-space, the sparsity of the peripheral sampling over time likely results in significant sampling mismatches across the respiratory cycle 11. However, compared to the TWIST and FLASH sequences, the radial sequence did demonstrate a slight decrease in SNR. This is likely due to the streaking artifacts due to under sampling or aliasing signal from coil elements outside the FOV, and may be reduced with post-processing 12 for under sampling and proper coil element selection for aliasing signal.CONCLUSIONS:
Compared to other commonly used DCE-MRI sequences, a free-breathing DCE-MRI sequence with Radial Stack of Stars k-space coverage provided dynamic images with minimal motion distortion. SNR and CNR were comparable among the sequences. Availability of a practical, motion-insensitive and free-breathing compatible DCE-MRI protocol will enable reproducible quantitative multiparametric pharmacokinetic evaluation and response assessment to novel therapeutics in clinical trials.Acknowledgements
T.S.C. Ng is supported by the BWH Radiology Residency.References
1. Chandarana, H.; Block, T. K.; Rosenkrantz, A. B.; Lim, R. P.; Kim, D.; Mossa, D. J.; Babb, J. S.; Kiefer, B.; Lee, V. S., Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Invest. Radiol. 2011, 46 (10), 648-53.
2. Sato, A. K.; Stevo, N. A.; Tavares, R. S.; Tsuzuki, M. S. G.; Kadota, E.; Gotoh, T.; Kagei, S.; Iwasawa, T., Registration of temporal sequences of coronal and sagittal MR images through respiratory patterns. Biomedical Signal Processing and Control 2011, 6 (1), 34-47.
3. Tavares, R. S.; de Sales Guerra Tsuzuki, M.; Gotoh, T.; Kagei, S.; Iwasawa, T., Lung Movement Determination in Temporal Sequences of MR Images Using Hough Transform and Interval Arithmetics. IFAC Proceedings Volumes 2009, 42 (12), 192-197.
4. Ceresoli, G. L.; Zucali, P. A., Anti-angiogenic therapies for malignant pleural mesothelioma. Expert opinion on investigational drugs 2012, 21 (6), 833-44.
5. Ceresoli, G. L.; Bonomi, M.; Sauta, M. G., Immune checkpoint inhibitors in malignant pleural mesothelioma: promises and challenges. Expert Rev. Anticancer Ther. 2016, 16 (7), 673-5.
6. Tokuda, J.; Mamata, H.; Gill, R. R.; Hata, N.; Kikinis, R.; Padera, R. F., Jr.; Lenkinski, R. E.; Sugarbaker, D. J.; Hatabu, H., Impact of nonrigid motion correction technique on pixel-wise pharmacokinetic analysis of free-breathing pulmonary dynamic contrast-enhanced MR imaging. J. Magn. Reson. Imaging 2011, 33 (4), 968-73.
7. Coolen, J.; De Keyzer, F.; Nafteux, P.; De Wever, W.; Dooms, C.; Vansteenkiste, J.; Roebben, I.; Verbeken, E.; De Leyn, P.; Van Raemdonck, D.; Nackaerts, K.; Dymarkowski, S.; Verschakelen, J., Malignant pleural disease: diagnosis by using diffusion-weighted and dynamic contrast-enhanced MR imaging--initial experience. Radiology 2012, 263 (3), 884-92.
8. Giesel, F. L.; Bischoff, H.; von Tengg-Kobligk, H.; Weber, M. A.; Zechmann, C. M.; Kauczor, H. U.; Knopp, M. V., Dynamic contrast-enhanced MRI of malignant pleural mesothelioma: a feasibility study of noninvasive assessment, therapeutic follow-up, and possible predictor of improved outcome. Chest 2006, 129 (6), 1570-6.
9. Giesel, F. L.; Choyke, P. L.; Mehndiratta, A.; Zechmann, C. M.; von Tengg-Kobligk, H.; Kayser, K.; Bischoff, H.; Hintze, C.; Delorme, S.; Weber, M. A.; Essig, M.; Kauczor, H. U.; Knopp, M. V., Pharmacokinetic analysis of malignant pleural mesothelioma-initial results of tumor microcirculation and its correlation to microvessel density (CD-34). Acad. Radiol. 2008, 15 (5), 563-70.
10. Zaitsev, M.; Maclaren, J.; Herbst, M., Motion artifacts in MRI: A complex problem with many partial solutions. J. Magn. Reson. Imaging 2015, 42 (4), 887-901.
11. Song, T.; Laine, A. F.; Chen, Q.; Rusinek, H.; Bokacheva, L.; Lim, R. P.; Laub, G.; Kroeker, R.; Lee, V. S., Optimal k-Space Sampling for Dynamic Contrast-Enhanced MRI with an Application to MR Renography. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2009, 61 (5), 1242-1248.
12. Lin, W.; Guo, J.; Rosen, M. A.; Song, H. K., Respiratory Motion-Compensated Radial Dynamic Contrast-Enhanced (DCE)-MRI of Chest and Abdominal Lesions. Magn. Reson. Med. 2008, 60 (5), 1135-1146.
Figures