2144
Optimizing data efficiency in SENCEFUL-based lung perfusion studies1Department of Diagnostic and Interventional Radiology, University Hospital of Würzburg, Würzburg, Germany
Synopsis
SElf-gated Non-Contrast-Enhanced FUnctional Lung imaging (SENCEFUL) allows assessment of lung ventilation and perfusion without the use of contrast agent or ionizing radiation. The original implementation, however, is rather inefficient in terms of data usage when reconstructing perfusion weighted datasets, as it analyzes data from a single breathing state only. In this study we present an approach that uses data from all breathing states, aiming at an improved quality of the resulting perfusion maps. A registration algorithm was applied for this purpose.
Introduction
SElf-gated Non-Contrast-Enhanced FUnctional Lung imaging (SENCEFUL) [1] is an extension to the standard Fourier decomposition method [2] and is capable of extracting perfusion weighted data from lung MRI by exploiting gating signals [3]. The reconstruction of perfusion weighted datasets by the SENCEFUL implementations presented up to now [4,5] is, however, not optimized in terms of data efficiency, as ~2/3 of the data are neglected.
Thus, it was the goal of this study to propose an updated SENCEFUL reconstruction with optimized data usage, and to investigate its impact on the perfusion maps.
Methods
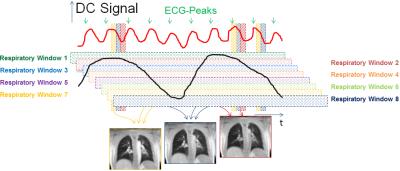
To reconstruct a perfusion weighted dataset in the conventional SENCEFUL approach, a defined window in the respiratory DC-signal (extracted from the signal of a coil near the diaphragm) is chosen (“Respiratory Window 1” in figure 1) and the cardiac DC-signal (extracted from the signal of a coil near the aorta) is used to resolve the cardiac cycle (vertical bars in figure 1) for perfusion assessment. This restriction leads to substantial amounts of acquired data being discarded in image reconstruction. The new approach now aims at exploiting data from the complete breathing cycle (all respiratory windows in figure 1) and reconstructs a cardiac cycle for eight different breathing states using a sliding window implementation. These different breathing states are registered to one reference breathing state using a non-rigid motion correction [6] and averaged subsequently. From this averaged data, perfusion maps were created using the absolute value of the first frequency component of the time series Fourier transform as proposed earlier [1,4]. Additionally, perfusion phase maps were calculated as the shift in phase of the first frequency component [4].
The proposed reconstruction was applied to previously acquired data from 4 healthy volunteers and 4 patients suffering from cystic fibrosis with the following parameters: TR: 2.5ms; TE: 0.7ms; flip angle: 8°; FOV: 450x450mm2; matrix size: 128x128; slice thickness: 10 mm; repetitions: 500. 64,000 phase encoding steps were acquired per slice in a period of 160s under free breathing.
The number of phase encoding steps used within the updated image reconstruction was compared to those used in the standard approach. For quality assessment of the artifact and noise level of the reconstructed datasets, the total variation (TV) in time was calculated. The peak-to-offset ratio was used as criterion for quality assessment of the perfusion phase map heterogeneity [5].
Results
The new approach used 392,150 ± 23,300 (mean ± SD) phase encoding steps to reconstruct an image series representing one cardiac cycle compared to 21,047 ± 4,090 for the conventional SENCEFUL approach.
For healthy volunteers, the relative total variation of the conventional approach was 2.7±1.8 (mean ± SD) times higher than the updated one. For patients, a factor of 2.5±1.3 was obtained. Peak-to-offset ratio of the perfusion phase maps increased by 92% and 38% for the patients and the healthy volunteers, respectively.
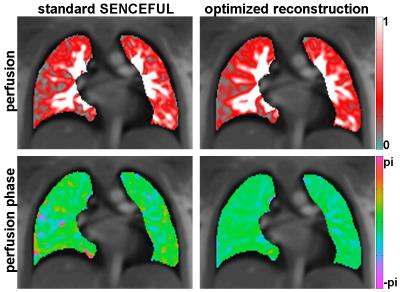
Figure 2 shows a comparison of perfusion (top) and perfusion phase maps (bottom) of a healthy volunteer, obtained by both approaches. A more homogeneous appearance of the maps after reconstruction with the new approach is clearly visible. For the presented example, relative total variation increased by a factor of 2.2 and the peak-to-offset ratio of the perfusion phase map of the presented volunteer was improved by 16%.
Discussion
The sheer increase of phase encoding steps used in the new approach resulted in superior image quality of the ECG resolved images, subsequently improving quality of the perfusion and perfusion phase maps. This was confirmed be the decrease in relative total variation of the reconstructions and the increase in the peak-to-offset ratio of the perfusion phase mapsConclusion
Using more data during image reconstruction in SENCEFUL results in vastly improved data efficiency. Moreover, quality of perfusion and perfusion phase maps could be improved.Acknowledgements
Grant sponsor: Siemens HealthcareReferences
[1] Fischer et al., NMR in Biomedicine, 27: 907-917 (2014)
[2] Bauman et al., Magn Reson Med, 62:656–664 (2009)
[3] Weick et al., J Magn Reson Imag, 37:727–732 (2013)
[4] Stäb et al., Proc. Int. Soc. Mag. Reson.
Med., 23:981 (2015)
[5] Veldhoen et al., Proc. Int. Soc. Mag. Reson. Med., 24:1144 (2016)
[6] Myronenko et al., IEEE Trans Med Imaging, 29:1882-1891 (2010)
Figures