2143
Dissolved phase Hyperpolarized Xenon-129 pulmonary imaging in the presence of gaseous Xenon signal1Department of Medical Physics, University of Wisconsin at Madison, Madison, WI, United States, 2Center for In Vivo Microscopy, Department of Radiology, Duke University Medical Center, Durham, NC, United States
Synopsis
Dissolved-phase hyperpolarized Xenon-129 imaging shows promise as a means to evaluate gas transfer from the airspaces of the lungs to parenchymal tissue and the blood stream. This typically requires selective excitation of dissolved-phase 129Xe, but its short T2* requires the use of short RF pulses. This reduces the achievable spectral selectivity and often leads to unwanted excitation of gas-phase Xenon. In this work, we present a method to selectively remove gas-phase contamination from dissolved-phase images. Our method is developed and validated with guidance from simulated data using a digital phantom and shown to be feasible in human subject scans.
Purpose
Imaging and separation of the dissolved-phase signal of hyperpolarized Xenon-129 (HP Xe-129) provides regional information on the transfer of gases in the lungs to the parenchymal tissue and blood stream[1, 2]. Dissolved-phase imaging is complicated by low spin density relative to the gas phase (1-2%) and short T2* of the dissolved-phase components (~2.1ms and ~2.5ms in red blood cells and lung tissues, respectively). This leads to competing demands for high spectral selectivity and short echo times (TE). We investigate the use of a short 1.2ms SLR pulse that allows gas-phase Xenon contamination of dissolved-phase images, and the feasibility of retrospective removal of the contaminant gas signal.Methods
The proposed workflow for estimation and removal of this gas-phase contamination is shown in Figure 1. The correction requires simultaneous acquisition of multi-TE gas- and dissolved-phase images and uses the following assumptions: (1) gas-phase contamination of the dissolved-phase may be estimated from gas-only data multiplied by a complex scale factor and modulated by the known off-resonance frequency, and (2) the gas-phase contamination of the dissolved-phase images can be isolated from dissolved-phase components by exploiting their large differences in T2*.
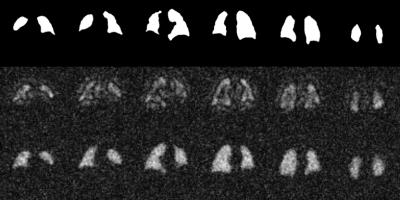
Simulation: To test our assumptions, a digital phantom was created and both the gas- and dissolved-phase signals were simulated based on a 3D radial acquisition (Figure 2). Data was simulated with varying amounts of gas contamination in the dissolved-phase signal over a range of SNR values and TEs.
Human Subject Imaging: Gaseous and dissolved-phase MRI was acquired at 1.5T in a single 15-s breathhold using 1L of HP 129Xe and a multi-echo 3D radial sequence. A 1.2ms SLR RF pulse with a 5 kHz bandwidth (+/- 2.5 kHz) was designed to have minimal stop band ripple (0.1%) in order to generally minimize off-resonance power. The first TE was calculated from an initial calibration spectrum such that the RBC and barrier components were 90 degrees out of phase (TE90), and the second TE was further delayed by 3.3ms. Transmit and receive frequencies were alternated between the gas and RBC resonance, acquiring the same projection angles in k-space for both frequencies, similar to Kaushik et. al. [3].
Results
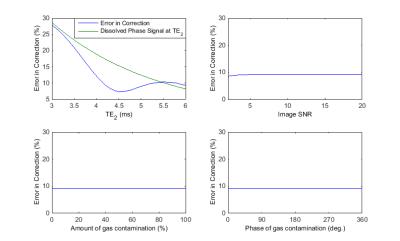
Simulation: Simulated data validates the efficacy of this procedure in removing the contaminant signal, as can be qualitatively observed in Figure 2. Simulations indicate that the error in the estimate of the gas contamination is strongly dependent on the amount of dissolved-phase signal remaining in the last acquired TE (Figure 3). Note the minimum in the error observed with second TE emerges due to phase cancellation in the two dissolved-phase components. Importantly, the error from gas contamination was found to be independent of the amplitude and phase of the gas contamination as well as SNR. These results indicate that gas-phase contamination is minimized to less than 10% of the dissolved-phase signal when TE2 is greater than 4.1ms.
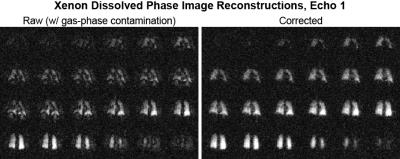
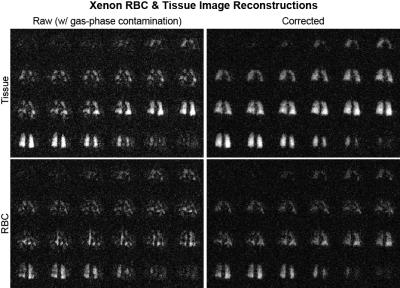
Imaging Data: Feasibility of the technique implemented in a human subject study showed apparent removal of the gas-phase contamination from dissolved component Xenon images. The resulting images qualitatively mirror the simulated results (Figure 2) and show an easily observable removal of apparent image artifacts (Figure 4). The removal of gas-phase contamination extends to improved conspicuity of the separate RBC and tissue component images (Figure 5).
Discussion
In our experience conducting HP 129Xe MR spectroscopic imaging studies of both gas and dissolved phases, significant gaseous Xenon is excited despite careful RF pulse design. Other groups have mitigated off-resonance gas contamination by ensuring the use of optimal center frequency and power settings that minimize power at the gas-phase resonant frequency. However, this approach requires very careful calibration of the stop-band attenuation characteristics of the pulse, which can constrain other critical pulse design elements (e.g. flip angle). For example, RF pulse and hardware specific system instability could lead to variable performance, such as in multi-scanner and multi-site studies. The proposed method provides a more general approach making for easier methodologic dissemination and replication. It also allows flexibility in sequence parameters, particularly RF frequencies and flip angles, and reduces the need for highly specific RF pulse design with consequent tradeoffs in achievable TE’s.
As the estimation of the magnitude of gas contamination is dependent on the dissolved-phase signal of the last echo, this technique could be further improved by extending the TE2 and allowing for further T2* decay of dissolved-phase signals.
Conclusion
We have demonstrated the effectiveness of estimation and removal of gas-phase contamination in dissolved-phase HP Xe-129 images.Acknowledgements
The authors would like to acknowledge support from the NIH/NHLBI R01 HL126771; R01HL105643; U01 HL109168 and GE Healthcare.References
1. Driehuys B, Cofer GP, Pollaro J, et al (2006) Imaging alveolar-capillary gas transfer using hyperpolarized 129Xe MRI. Proc Natl Acad Sci U S A 103:18278–18283. doi: 10.1073/pnas.0608458103
2. Cleveland ZI, Cofer GP, Metz G, et al (2010) Hyperpolarized 129Xe MR Imaging of Alveolar Gas Uptake in Humans. PLoS ONE 5:e12192. doi: 10.1371/journal.pone.0012192
3. Kaushik SS, Robertson SH, Freeman MS, et al (2016) Single-breath clinical imaging of hyperpolarized (129)Xe in the airspaces, barrier, and red blood cells using an interleaved 3D radial 1-point Dixon acquisition. Magn Reson Med 75:1434–1443. doi: 10.1002/mrm.25675
4. Mugler J, Altes T, Ruset I, et al Image-based measurements of T2* for dissolved-phase Xe129 in the human lung (abstract). In: Proc. 20th Annu. Meet. ISMRM. Melbourne, p 1347
Figures