2142
Assessment of Repeatability of Disease Burden and ADC estimates in Malignant Pleural Mesothelioma using Diffusion Weighted Imaging1Division of Radiotherapy and Imaging, Cancer Research UK Cancer Imaging Centre, Institute of Cancer Research, London, United Kingdom, 2Radiology, Royal Marsden Hospital, London, United Kingdom
Synopsis
We demonstrate the repeatability of tumour volume and apparent diffusion coefficient (ADC) estimates; obtained by combining 3D semi-automatic segmentation with a global ADC threshold using DW-MRI in malignant pleural mesothelioma. The results of our classification of solid tumour show excellent repeatability of mean and median ADC estimates and tumour volume. Our methodology provides a clinical tool for radiologists to evaluate tumour burden of MPM in a fast and highly repeatable way.
Background
In malignant pleural mesothelioma (MPM), assessing tumour volume and treatment response accurately is challenging due to the unusual growth pattern and the presence of both solid disease and pleural effusion and the presence of motion1. In previous studies, malignant pleural mesothelioma in diffusion-weighted imaging were analysed as a whole tumour, however, solid disease is the key factor to response of the anti-tumour treatment1,2.Purpose
To evaluate the repeatability of tumour volume and apparent diffusion coefficient (ADC) metrics using a combination of 3D semi-automatic segmentation and global thresholding of ADC estimates from DW-MRI in MPM.Methods
Patients: Six patients with histopathologically proven MPM in a current prospective clinical trial were recruited and scanned twice (interval between 1 hour and 7 days) before starting therapy.
Imaging protocol: Diffusion-weighted spin-echo EPI was acquired using a 1.5T scanner (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany) using two body-array surface receiver coils and spine matrix. Two imaging volumes (covering the whole chest) were used: 30 axial slices/volume, slice thickness 5mm, TR/TE=9000/82 ms, b =100/500/800 s/mm2 (3-Scan-Trace), resolution = 3×3mm2, FOV = 273×380 mm, matrix = 92×128, NSA = 4, receive bandwidth = 1860 Hz/pixel, parallel acquisition (Grappa acc. factor 2, ref lines 30); fat Suppression: SPAIR; free breathing; acquisition time ~6 min/volume.
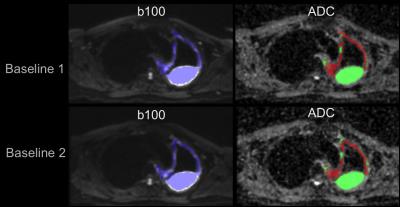
Data analysis: The whole tumour volume was segmented by using a 3D semi-automatic tool (in-house software3, 4, 5) with back- and fore-ground seeds drawn on the normal tissue and tumour tissue respectively on the mean b-value 100 s/mm2 images. b100 s/mm2 images were chosen as both solid tumour and pleural effusions had the highest signal intensities whilst maintaining good disease/background contrast among all DW images. Segmented ROIs were then transferred to calculated ADC maps. Solid tumours and pleural effusions were classified below and above an ADC threshold of 2000*10-6mm2/s respectively within the whole tumour volume for each patient. The repeatability of parameters (mean/ median ADC, and tumour volume) of the whole tumour and solid component in the two baseline measurements was evaluated by using the Bland-Altman method. The median ADC value from all pixels in the tumour volumes was used to reduce the sensitivity to outliers. The Coefficient of Variation (CV) of the log-transformed data (ADC/volumes) was used to measure the repeatability of the parameters:
$$CV = 100\% \times \sqrt{exp \left( \left( \sum d^2 \right) /2N \right)-1}$$
where d is the difference of the log-transformed paired baseline measurements and N is the number of patients.
Results
Segmented whole tumour regions (Blue region) showed a good visual match with both the low b-value diffusion-weighted images and the ADC maps in the repeated pre-treatment scans (Figure 1).
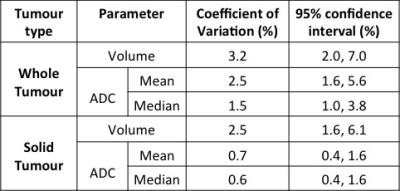
CVs of ADC (mean and median) and tumour volumes and their 95% confidence intervals are shown in Table 1, with CVs of 3.2% or lower for all parameters. This indicates a good repeatability of our proposed method, in particular for solid tumour with CVs less than 2.5%.
The lower and upper 95% limits of agreement (LoA) of the whole tumour volume in this study are -8.4%, and 9.1% respectively.
Discussion
After applying the 3D semi-automatic segmentation, the segmented tumour regions match in both the low b-value images and ADC. In terms of tumour burden and ADC, the repeatability of the solid tumour and whole tumour is good. The worst case (top of range at 95% confidence interval) of the CV is only 7%. Low CVs (0.6 to 3.2%) and 95% LoA mean that it will be less likely to undermine the treatment effects or disease heterogeneity when applying our methodology. Our method offers a clinical tool for radiologists to evaluate the tumour burden of MPM in a fast and highly repeatable way. The limitation of this pilot study is the small sample size and a larger patient cohort will be investigated in future studies.Conclusion
The classification of solid tumour by using a 3D semi-automatic segmentation method and a global ADC threshold shows excellent repeatability of mean and median ADC estimates and tumour volume in the diffusion-weighted imaging.Acknowledgements
CRUK and EPSRC Cancer Imaging Centre in association with the MRC and Department of Health grant C1060/A10334; NHS funding to the NIHR Biomedical Research Centre and the Clinical Research Facility and post-doctoral fellowship funding by the NIHR (NHR011X); An Experimental Cancer Medicine Centre Network award (C51/A7401 & C12540/A15573); British Lung Foundation grant (APP13-4). Martin O Leach is a NIHR Emeritus Senior Investigator.References
1. Cheng, L. et al. Response evaluation in mesothelioma: Beyond RECIST. Lung Cancer 90.3 (2015): 433-441.
2. Gill, RR., et al. Diffusion-weighted MRI of malignant pleural mesothelioma: preliminary assessment of apparent diffusion coefficient in histologic subtypes. American Journal of Roentgenology 195.2 (2010): W125-W130.
3. Vezhnevets, V. and Konouchine V., Proc. Graphicon, (2005): 150-156.
4. Blackledge MD et al., Rapid development of image analysis research tools: Bridging the gap between researcher and clinician with pyOsiriX, Comp. Biol. Med., 69(2016): 203-212.
5. Blackledge, MD, et al. Computed diffusion-weighted MR imaging may improve tumor detection. Radiology 261.2 (2011): 573-581.
Figures