Lucia Flors1, Talissa A Altes2, John P Mugler III1, G Wilson Miller1, Jaime F Mata1, Sarah K Kilbourne3, Hannah C Mannem3, Max M Weder3, and Yun M Shim3
1Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States, 2Department of Radiology, University of Missouri, 3Pulmonary and Critical Care Medicine, University of Virginia
Synopsis
Purpose: To determine if the changes in
lung ventilation using HP 3He-MRI can provide in vivo pulmonary physiology
highly relevant in defining CLAD phenotypes among lung transplant patients,
phenotypes which otherwise are undetectable by the usual PFT parameters such as
FEV1.
Methods: Thirteen lung transplant
recipients underwent ventilation HP 3He MR lung imaging and spirometry; the
latter was compared to baseline spirometry. Time from transplant was 2.5 ±2.5
yrs.
Results/ Conclusion: Declined lung
function after lung transplant correlated well with decreased ventilated lung
volume in the transplanted lung found with HP 3He MRI.
Purpose
Lung transplant is the only
definitive treatment in patients with end-stage lung disease. Chronic allograft
rejection is the major cause of long-term morbidity and mortality in lung
transplant recipients. The traditionally described chronic rejection, bronchiolitis
obliterans syndrome (BOS), manifests as a persistent fall in forced expiratory
volume in one second (FEV1) and associated ventilatory defects. However,
recently a restrictive form of chronic rejection has been described that does
not fit the traditional definition of BOS. The term chronic lung
allograft dysfunction (CLAD) has been introduced to include all forms of graft
dysfunction. Pulmonary function tests (PFT) provide global information about the
native and transplanted lung. FEV1 and forced vital capacity (FVC) are reproducible,
but neither are sensitive nor specific. Computed tomography (CT) provides structural
information but often shows few or even no changes, despite clinical deterioration
of the patient’s condition. With hyperpolarized helium-3 (HP 3He) MR imaging, individual
lung function can be assessed; the regional ventilation can be depicted without onizing radiation and thus scanning can be repeated in the same
subject without significant risk. HP 3He-MRI
has been shown to be a sensitive method to detect ventilatory defects in lung
transplant recipients (1,2). Hence, the purpose of this study
was to determine if the changes in lung ventilation using HP 3He-MRI can
provide in vivo pulmonary physiology
highly relevant in defining CLAD phenotypes among lung transplant patients, phenotypes
which otherwise are undetectable by the usual PFT parameters such as FEV1.Methods
Thirteen lung transplant recipients [55.1
±7.1 yrs (range 43, 68 yrs)] were included in the study under a protocol
approved by our institutional review board. Informed consent was signed by the
patients. Indications for transplant were: 7 patients with COPD, 1 with Langerhans
Cell Histiocytosis, 1 pulmonary hypertension, 1 alpha antitrypsin deficiency, 2
idiopathic pulmonary fibrosis and 1 with cystic fibrosis. Eleven patients had
undergone unilateral lung transplant (7 right, 4 left) and two patients had
undergone bilateral lung transplant. Time from transplant to inclusion in the
study was 2.5 ±2.5 yrs (range 0.25- 9.9 yrs).
MR imaging was performed using a commercial 1.5-T
whole-body scanner (Vision[ 1] , Siemens Medical Solutions, Malvern, PA) modified to
operate at the 3He resonant frequency of 48 MHz by the addition of a broadband
radiofrequency amplifier and a flexible 3He chest radiofrequency coil (IGC
Medical Advances, Milwaukee, WI). Contiguous axial MR images covering the
entire lung (FLASH; TR/TE, 7/3 ms; flip angle, 10°; matrix, 80x128; FOV, 26 x
42 cm; section thickness, 10 mm; interslice gap, none) were collected during a
15-20 sec breath hold immediately following inhalation of approximately 300 mL
H3He gas mixed with approximately 700 mL of nitrogen. For each scan, the ventilated lung volume (%VD) on 3He-MRI was manually
segmented by two independent blinded readers.
Spirometry was performed in all
patients at the time of the MRI exam and was compared to the baseline
spirometry performed at the time of the transplant (%FEV1 change = %FEV1 at
baseline - %FEV1 at the time of the study). % FEV1 change was annualized by dividing it by
the number of years since the transplant.
Spearman correlation between the ventilated volume of the
transplanted lung and %FEV1 change (both
absolute and annualized %FEV1 change) was calculated.
Results
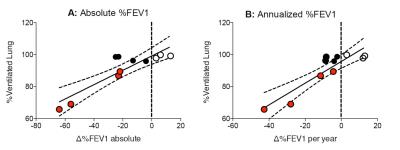
Absolute
%FEV1 change was -19.8±26.6% (range -64, +13). Annualized %FEV1 was -8.9±18.1% (range -42.7,
+13). %VD in the transplanted lung was: 1) reader 1: 90.8%±12.8% (range 99.94-64.8),
2) reader 2: 87.8±14.01% (range 99.85-59.7), 3) average: 89.3±13.3 % (range 99.83-65.8). There was significant
correlation between the absolute %FEV1 reduction and %VD (r=0.8364,
p=0.0022, Fig 1a) as well as between annualized %FEV1 reduction and %VD (r=0.6925,
p=0.0215, Fig 1b). Of note, 3He-MRI identified highly interesting CLAD
phenotypes. While %VD was reduced in 4 subjects (solid red circles)
with matching drop in %FEV1 and %VD, four other subjects (solid black circles) had divergent
%FEV1 and %VD, dropping
%FEV1 but normal %VD. These
divergent patterns of the %FEV1 and %VD were highly suggestive of restrictive CLAD found
in the four subjects (solid black circle) instead of classic BOS noted in the other
four subjects (solid red circles).
Discussion
Declined
lung function after lung transplant correlated well with decreased ventilated
lung volume in the transplanted lung found with HP 3He-MRI. The discrepancy
found in patients which showed %FEV1 reduction without impaired ventilation on
HP 3He-MRI may indicate that the transplanted lung is experiencing both obstructive
and restrictive disease, and therefore this imaging technique has the potential
to provide new insights into the pathologic changes of chronic transplant
rejection, currently termed CLAD.Acknowledgements
No acknowledgement found.References
[1] McAdams HP, et al.
AJR Am J Roentgenol 1999 173:955–959. [2] Gast KK, et al. J Magn Reson Imaging. 2002 Mar;15(3):268-74