2120
Dynamic Contrast-Enhanced Breast MRI using A Chemically Fat-Suppressed View-Sharing Technique1Global MR Applications & Workflow, GE Healthcare, Madison, WI, United States, 2Global MR Applications & Workflow, GE Healthcare, Hino, Japan, 3Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 4Radiology, University of Wisconsin-Madison, Madison, WI, United States, 5Global MR Applications & Workflow, GE Healthcare, Houston, TX, United States, 6Carbone Cancer Center, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
In clinical breast MRI, the dynamic contrast-enhanced (DCE) T1-weighted fat-suppressed scan plays an essential role for lesion detection and characterization. In order to improve temporal resolution of the dynamic scan, view-sharing techniques are typically used along with Dixon-based water-fat separation methods. However, there are several limitations and drawbacks of using Dixon-based techniques. In this work, we proposed to use chemical fat suppression with view-sharing to improve the temporal resolution of DCE breast MRI.
INTRODUCTION
In clinical breast MRI, the dynamic contrast-enhanced (DCE) T1-weighted fat-suppressed scan plays an essential role for lesion detection and characterization1. High spatial resolution is necessary for the assessment of lesion morphology1. However, scan times are long, which makes it clinically unreliable to capture contrast dynamics to aid lesion characterization. To improve temporal resolution and maintain fat suppression capability, view-sharing techniques have been proposed2-5, and are typically used in combination with multi-echo Dixon-based methods for fat suppression/separation6,7. However, there are limitations and drawbacks of multi-echo Dixon-based approaches, such as longer TR or longer scan time due to the need to collect multiple echoes and higher demand for gradient hardware if bipolar readout is used, especially at 3T. One alternative approach is to use spatial-spectral RF excitation (i.e. water-excitation), but there is a significant scan time penalty due to the long RF pulse, especially at 1.5T. In this work, we proposed the use of single-echo chemical fat suppression with view-sharing to improve the temporal resolution for high spatial resolution breast DCE MRI. Specifically, the quality of the fat suppression using the proposed method was compared to standard non-view-shared fat suppression sequence, and the impact on contrast dynamics was analyzed.METHODS
A fully-sampled ky-kz plane is first accelerated using partial Fourier and conventional data-driven parallel imaging8. For view-sharing, k-space is divided into a central region (A region) and multiple outer sub-regions (B regions, named as B1, B2, B3, …). Each Bi (i = 1, 2, 3, …) sub-region pseudo-randomly sub-samples the outer k-space in an interleaved fashion, which is needed for subsequent view-sharing reconstruction. Next, further segmentation of k-space is performed such that each chemical fat suppression pulse is played out and followed by the acquisition of a segment of k-space views, which contains points from both central A region and one outer Bi sub-region. The entire acquisition scheme will look like the following:
- First set of segments, with each segment containing one chemical fat suppression pulse followed by ky-kz views from a subset of A and a subset of B1
- Second set of segments, with each segment containing one chemical fat suppression pulse followed by ky-kz views from a subset of A and a subset of B2
- Third set of segments, with each segment containing one chemical fat suppression pulse followed by ky-kz views from a subset of A and a subset of B3
- Fourth set of segments, with each segment containing one chemical fat suppression pulse followed by ky-kz views from a subset of A and a subset of B1 (assuming there are only 3 outer Bi sub-regions)
- …
In the reconstruction, like conventional view-sharing reconstruction, each A region will be combined with its neighboring B sub-regions to form a parallel-imaging-ready k-space data set, and then reconstructed with conventional data-driven parallel imaging.
Two healthy volunteers were recruited and scanned on a 3.0T scanner (Discovery MR 750w, GE Healthcare, Waukesha, WI, U.S.A.) using a 8-channel breast coil (GE Healthcare). Scanning parameters included: axial field-of-view = 32 × 32 cm2, matrix size 448 × 448, 138 slices with 1.4mm thickness (then interpolated to 276 slices with 0.7mm thickness), flip angle = 10 degrees, BW = ±62.5 kHz, parallel imaging factor of 2 × 1 = 2. An adiabatic fat saturation pulse was used. Each volunteer received a single weight based dose (0.1 mmol/kg) of gadobenate dimeglumine (Multihance) power injected at 2 cc/sec followed by a 20 cc saline flush. One mask (non-view-shared) phase, four view-shared phases and two delayed non-view-shared phases were acquired. Each non-view-shared phase is 3 min, and each view-shared phase is 1 min. For comparison of fat suppression quality, a conventional chemical fat suppression sequence (incompatible with view-sharing) was obtained at the end.
RESULTS
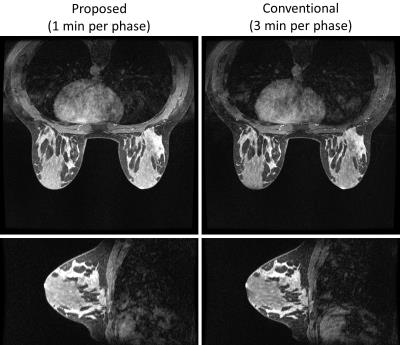
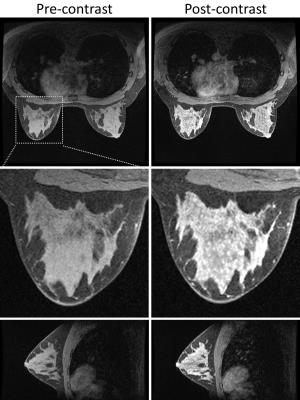
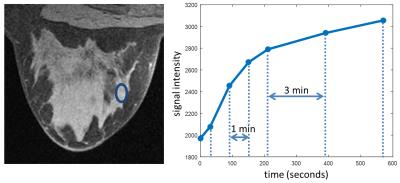
Figure 1 shows the comparison of fat suppression between the proposed method and the conventional method for the first volunteer. Both methods show similar SNR level and good homogeneity of the fat suppression. Figure 2 shows the pre- and post-contrast images of the second volunteer from the proposed method, with very high spatial resolution and good depiction of the anatomical details. Figure 3 shows the contrast dynamics curve of a selected ROI.CONCLUSION
In this work, chemical fat suppression was combined with a view-sharing technique to achieve about 3x higher temporal resolution for DCE breast MRI while maintaining similar SNR level and good fat suppression quality. Contrast temporal dynamics were captured and the quality and the homogeneity of the fat suppression were similar to that of a conventional non-view-sharing sequence.Acknowledgements
No acknowledgement found.References
1. Kuhl CK, Schild HH, Morakkabati N. Dynamic bilateral contrast-enhanced MR imaging of the breast: Tradoff between spatial and temporal resolution. Radiology. 2005;236(3):789-800.
2. Korosec FR, Frayne R, Grist TM and Mistretta CA. Time-resolved contrast-enhanced 3D MR angiography. Magn Reson Med. 1996;36(3):345-351.
3. Haider CR, Hu HH, Campeau NG, Huston J and Riederer SJ. 3D high temporal and spatial resolution contrast-enhanced MR angiography of the whole brain. Magn Reson Med. 2008;60(3): 749-760.
4. Saranathan M, Rettmann DW, Hargreaves BA, Clarke SE, Vasanawala SS. Differential subsampling with cartesian ordering (DISCO): a high spatio-temporal resolution dixon imaging sequence for multiphasic contrast enhanced abdominal imaging. J Magn Reson Imaging. 2012;35(6):1484-1492.
5. Lim RP, Shapiro M, Wang EY, et al. 3D time-resolved MR angiography (MRA) of the carotid arteries with time-resolved imaging with stochastic trajectories: comparison with 3D contrast-enhanced bolus-chase MRA and 3D time-of-flight MRA. Am J Neuroradiol. 2008;29:1847-1854.
6. Ma J. Breath-hold water and fat imaging using a dual-echo two-point dixon technique with an efficient and robust phase-correction algorithm. Magn Reson Med. 2004;52(2):415-419.
7. Eggers H, Brendel B, Duijndam A and Herigault G. Dual-echo Dixon imaging with flexible choice of echo times. Magn Reson Med. 2011;65(1):96-107.
8. Brau ACS, Beatty PJ, Skare S and Bammer, R. Comparison of reconstruction accuracy and efficiency among autocalibrating data-driven parallel imaging methods. Magn Reson Med. 2008;59(2):382-395.
Figures