2116
Advanced Ultrafast Dynamic Contrast Enhanced Breast MRI with Compressed Sensing VIBE1Radiology and Nuclear Medicine, Radboud University Medical Centre, Nijmegen, Netherlands, 2MR-Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Previous work showed that ultrafast breast DCE-MRI enables assessment of the contrast inflow curve while providing images at diagnostic spatial resolution. However, the slice thickness (~2.5mm) prevented multiplanar reconstructions and therefore did not yield the same morphological information as obtained with conventional T1-weighted series. We evaluate a compressed sensing VIBE sequence (CS-VIBE) for ultrafast breast MRI that enables high spatio-temporal resolution for both dynamic inflow analysis and morphological evaluation. Two reader-studies were conducted to evaluate image quality and lesion morphology assessment. Our results show that CS-VIBE combines the advantages of both high-spatial and high-temporal resolution of clinically available sequences.
Introduction
Dynamic Contrast-Enhanced (DCE) breast MRI is the most sensitive imaging method for early breast cancer detection.1 Regular screening protocols are, however, too costly to be offered to women without a hereditary breast cancer risk.2 This has led to the development of abbreviated protocols that, in their simplest form, discard all dynamic information and just show subtractions and maximum intensity projections of initial enhancement.3 Ultrafast MRI protocols re-enable dynamic analysis during the contrast inflow phase without lengthening the protocol. Previous studies with a TWIST (time-resolved angiography with stochastic trajectories) sequence4 showed that images with a diagnostic spatial resolution can be obtained in 4.3s per time-point, and the entire dynamic protocol can be obtained in an acquisition time of 102s. The dynamic parameters derived from this sequence, maximum slope and time-to-enhancement, achieve even better discrimination between benign and malignant lesions than conventional dynamic analysis.4,5
The downside of TWIST is that viewsharing gives rise to temporal blurring and, while the through-plane resolution (2.5mm) is within margins of current guidelines, it does not allow image reconstruction in other planes.
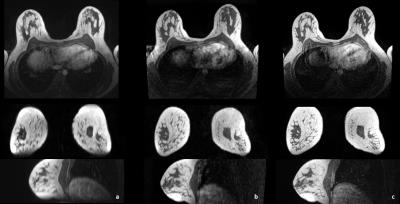
We explore a novel approach to ultrafast dynamic breast MRI using compressed sensing.6 This approach (CS-VIBE) allows a higher spatial resolution than TWIST by preserving temporal resolution, and therefore enables not only dynamic analysis but also multiplane reconstruction. We compare image quality (IQ) to TWIST and compare lesion conspicuity in CS-VIBE to that in routine T1-weighted DIXON-acquisitions of 1:31 min acquisition duration.
Methods
From 04/2016 to 10/2016, 30 women were included. The need for informed consent was waived. Women were scanned with a hybrid protocol on a 3T MAGNETOM Skyra system (Siemens Healthcare, Erlangen, Germany) using a 16-channel bilateral breast coil. The regular high-temporal-resolution TWIST sequence obtained during inflow of contrast (TR: 4.09ms, TE: 2.06ms, FA: 20°, spatial resolution: 0.9x0.9x2.5mm, temporal resolution: 4.57s) was replaced by the CS-VIBE prototype sequence (TR: 4.47ms, TE: 2.06ms, FA: 15°, spatial resolution: 0.8x0.8x1.6mm, temporal resolution: 4.47s) with continuous data acquisition for 1:40min. Subsequently, the standard T1-sequence, that was also obtained prior to contrast administration, was repeated (VIBE-DIXON; TR: 5.68ms, TE1/2: 2.46/3.69ms, FA: 15°, spatial resolution: 0.8x0.8x1.0mm, temporal resolution: 1:31min).
We conducted two reader-studies. The first study was conducted in 23 women in whom prior MRI exams with TWIST were available. Overall IQ of the CS-VIBE and TWIST images was independently rated by one experienced breast radiologist on a continuous scale from 1 to 100. In addition, the effect of several types of artefacts (e.g. motion, breathing and pulsation, ghosting and infolding, external influences) was rated on a five-point scale (1=heavily disturbing to 5=not present).
The second study was conducted using only women with lesions. In total, 31 lesions (4 malignant, 27 benign) were described in the clinical MRI reports. These lesions were independently evaluated on the CS-VIBE and the VIBE-DIXON sequence. Reporting (shape, margin, and internal enhancement features) was performed according to the BI-RADS lexicon.7 In addition, a lesion conspicuity score (1=not visible to 5=very clear) was given for each lesion on the two sequences. Statistical analysis was performed in SPSS, Wilcoxon signed-rank tests and paired t-tests were used for comparison.
Results
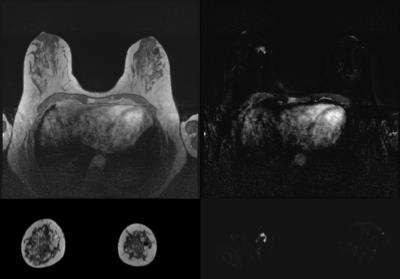
Results are presented in Table 1 and Table 2. Overall IQ was not significantly different between TWIST and CS-VIBE (p=0.062). While TWIST suffered more from ghosting- and infolding-artefacts (p<0.001), in CS-VIBE breathing- and pulsation-artefacts were stronger (p=0.001). One remaining artefact for CS-VIBE was recognized (as displayed in Figure 1), however, as this was always outside the area of interest, this was not considered a problem for evaluation of the MR images. Morphological lesion description using CS-VIBE was virtually equal to lesion description using the VIBE-DIXON. Also lesion conspicuity scores were similar (p=0.157). The reconstruction in different planes for every sequence is presented in Figure 2.Discussion
CS-VIBE enables ultrafast dynamic acquisitions in breast MRI at high spatial resolution and without temporal blurring. CS-VIBE demonstrated equivalent IQ compared to TWIST, and, in addition, resulted in similar lesion conspicuity and morphological assessment as the VIBE-DIXON acquisition. On the standard scanner hardware, reconstruction of the complete CS-VIBE time series per subject still takes ~45min. Future optimization of the reconstruction process will render the technique clinically viable. The evaluation of the dataset by additional readers is pending.Conclusion
CS-VIBE is a promising technique for ultrafast, high spatio-temporal resolution breast MRI. It enables the evaluation of inflow dynamics and multi-planar assessment of lesion morphology.Acknowledgements
No acknowledgement found.References
1. Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246(1):116-24.
2. Saadatmand S, Tilanus-Linthorst MM, Rutgers EJ, et al. Cost-effectiveness of screening women with familial risk for breast cancer with magnetic resonance imaging. Journal of the National Cancer Institute. 2013;105(17):1314-21.
3. Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(22):2304-10.
4. Mann RM, Mus RD, van Zelst J, Geppert C, Karssemeijer N, Platel B. A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Investigative radiology. 2014;49(9):579-85.
5. Tudorica LA, Oh KY, Roy N, et al. A feasible high spatiotemporal resolution breast DCE-MRI protocol for clinical settings. Magn Reson Imaging. 2012;30(9):1257-67.
6. Nickel D, Chen X, Mailhe B, et al. Motion-resolved 3D dynamic contrast enhanced liver MRI. Proc Intl Soc MRM 24(2016);4253
7. ACR BI-RADS Atlas. 5th ed, 2013.
Figures