2115
Comparison of methods for high spatial-resolution breast diffusion imaging1Department of Biomedical Engineering, University of Minnesota, Minneapolis, MN, United States, 2Department of Radiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
Diffusion weighted imaging (DWI) has applications in the screening, diagnosis, and treatment monitoring of breast cancer, but its clinical value in is limited by the low resolution and artifacts of standard methods. In this work we compared a standard method with two high-resolution techniques: read-out segmented EPI (RS-EPI) and single-shot simultaneous multi-slice EPI with in-plane slice encoding (SMS-IPSE). Both the SMS-IPSE and RS-EPI methods can produce high-resolution, accurate diffusion-weighted images at the cost of decreased SNR within a clinically practical 5-minute time window, enabling the detection of smaller lesions.
Background and Purpose
Diffusion weighted imaging (DWI) is commonly used in breast MR scans to measure the apparent diffusion coefficient (ADC) in lesions. Low ADC values are associated with malignancies, and increasing ADCs during chemotherapy can indicate response prior to conventional anatomical measurements (1). However, the clinical value of DWI is limited by its low spatial resolution and the artifacts associated with standard single-shot spin-echo echo planar imaging (SE-EPI) DWI methods, including B0 distortion, Nyquist ghosts, and chemical shift displacement. A number of groups have used alternative breast DWI methods with higher spatial resolution to better assess small lesions and compare with anatomical imaging (2-5). The purpose of this work is to compare the imaging performance of two high-resolution imaging approaches to a conventional DWI protocol using both phantom measurements and human subjects.Methods
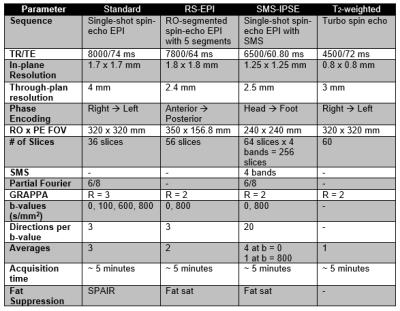
All measurements were performed on a Siemens 3 T PrismaFit scanner using a 16-channel Sentinelle breast coil. Three protocols using different sequences were configured to acquire bilateral axial ADC measurements within a time constraint of approximately 5 minutes (details Table 1). The standard protocol was based on the ACRIN 6698 clinical trial (6) and used a single-shot SE-EPI axial acquisition with nominal resolution 1.7 x 1.7 x 4 mm (11.6 μL). The RS-EPI protocol was based on Wisner et al.’s implementation of readout segmented EPI (4, 7) with 5 readout (RO) segments and nominal resolution 1.8 x 1.8 x 2.4 mm (7.8 μL). The third protocol used simultaneous multi-slice (SMS) and a strategy termed in-plane slice encoding (IPSE), in which slice-encoding is used for high-resolution encoding in the primary viewing plane, and phase encoding is used in the lower-resolution through-plane direction (8-10). This SMS-IPSE protocol had a nominal resolution of 1.25 x 1.25 x 2.5 mm (3.9 μL). A standard anatomical T2-weighted image was also acquired.
To compare spatial resolution and validate ADC values, phantom measurements were performed using a breast phantom featuring multiple ADC compartments, resolution grids, and solutions relaxation-matched to breast tissues (11,12). In vivo studies were acquired on seven normal volunteers and two patients under IRB-approved protocols.
Results
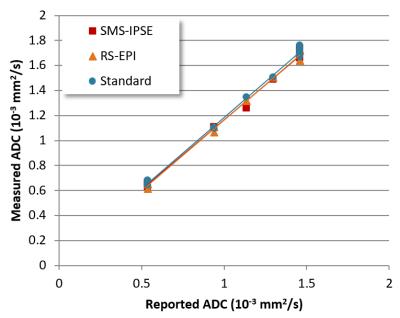
Phantom measurements: A comparison of the resolution with all methods is given in Figure 1. The SMS-IPSE protocol demonstrated resolution comparable to the T2-weighted anatomical image. The smallest detectable feature size in the SMS-IPSE image is 0.5 mm, compared to 0.75 mm in both standard and RS-EPI images. All three protocols measure similar ADC values (Figure 2).
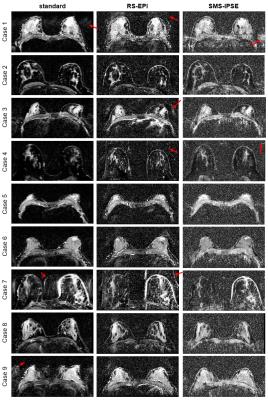
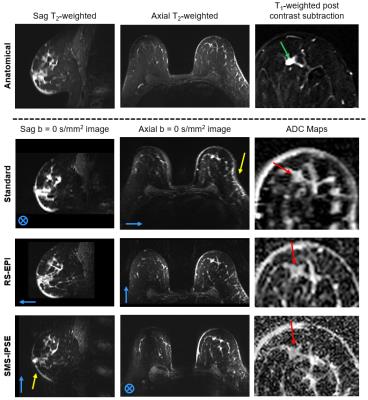
In vivo measurements: Figure 3 compares ADC maps across all methods for the nine subjects. Overall, SMS-IPSE provides finer spatial detail but substantially lower SNR than both RS-EPI and the standard method. All three methods have Nyquist ghosts in some cases. Figure 4 shows an example from a patient with a malignant lesion of 8 mm in its longest dimension. The in vivo resolution of the SMS-IPSE protocol is comparable to that of the T2-weighted anatomical image. In the ADC maps, the lesion is not easily detectible using standard methods due to partial volume effects and low resolution. While the lesion is visible in both the RS-EPI and SMS-IPSE methods, the SMS-IPSE ADC map most clearly characterizes the lesion with a low ADC, whereas RS-EPI is hindered by partial volume effects.
Discussion
High spatial resolution is important for clinical applications of DWI because it enables an accurate visualization and characterization of small lesions and foci. The SMS-IPSE method was able to reach a substantially higher resolution within similar time constraints as RS-EPI and the standard method because it uses more efficient spatial encoding. This resolution gain, however, comes as the expense of SNR loss. The SMS-IPSE is also prone to increased respiratory motion artifacts due to the IPSE reformatting scheme, where motion between slices becomes evident in the axial plane. Both SMS-IPSE and standard protocols show chemical shift displacement artifacts and geometric distortions caused by B0 inhomogeneity in their phase-encode directions; in comparison the RS-EPI images show small geometric distortions due to its short echo spacing. Distortion correction methods, which were not used in this work, can reduce these distortions but require additional scans to estimate the B0 offsets (13-16).Conclusions
Both the SMS-IPSE and RS-EPI methods can produce high-resolution, accurate diffusion-weighted images at the cost of decreased SNR within a clinically practical 5-minute time window. Further work is needed to improve artifact suppression and assess the clinical utility of both methods in patients.Acknowledgements
NIH P41 EB015894
NIH R21 CA 201834
References
1. Partridge SC, McDonald ES. Diffusion weighted MRI of the breast: Protocol optimization, guidelines for interpretation, and potential clinical applications. MRI Clin. 2013, 21(3):601-624.
2. Singer L, Wilmes LJ, Saritas EU, Shankaranarayanan A, Proctor E, Wisner DJ, Chang B, Joe BN, Nishimura DG, Hylton NM: High-resolution diffusion-weighted magnetic resonance imaging in patients with locally advanced breast cancer. Acad Radiol 2012, 19:526–534.
3. Wilmes LJ, McLaughlin RL, Newitt DC, Singer L, Sinha SP, Proctor E, Wisner DJ, Saritas EU, Kornak J, Shankaranarayanan A, Banerjee S, Jones EF, Joe BN, Hylton NM: High-Resolution Diffusion-Weighted Imaging for Monitoring Breast Cancer Treatment Response. Academic Radiology 2013, 20:581–589.
4. Wisner DJ, Rogers N, Deshpande VS, Newitt DN, Laub GA, Porter DA, Kornak J, Joe BN, Hylton NM: High-resolution diffusion-weighted imaging for the separation of benign from malignant BI-RADS 4/5 lesions found on breast MRI at 3T: DWI on Suspicious Breast Lesions at 3T. Journal of Magnetic Resonance Imaging 2014, 40:674–681.
5. Barentsz MW, Taviani V, Chang JM, Ikeda DM, Miyake KK, Banerjee S, van den Bosch MAA., Hargreaves BA, Daniel BL: Assessment of tumor morphology on diffusion-weighted (DWI) breast MRI: Diagnostic value of reduced field of view DWI. J Magn Reson Imaging 2015.
6. Hylton N, Partridge SC, Rosen M, and Chenevert T., Diffusion Weighted MR Imaging Biomarkers for Assessment of Breast Cancer Response to Neoadjuvant Treatment: A sub-study of the I-SPY 2 TRIAL. ACRIN. https://www.acrin.org/6698_protocol.aspx
7. Porter, DA, and Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magnetic Resonance in Medicine 2009, 62:468–475.
8. Larkman DJ, Hajnal JV, Herlihy AH, Coutts GA, Young IR, Ehnholm G. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. Journal of Magnetic Resonance Imaging. 2001, 13(2):313–7.
9. Sotiropoulos SN, Jbabdi S, Xu J, Andersson JL, Moeller S, Auerbach EJ, Glasser MF, Hernandez M, Sapiro G, Jenkinson M, Feinberg DA, Yacoub E, Lenglet C, Van Essen DC, Ugurbil K, Behrens TEJ. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. NeuroImage. 2013, 80:125–43.
10. Bolan PJ, Moeller S, Metzger GJ, Auerbach EJ, Lenglet C, Wang D, Kollasch P, Deshpande V, McKay JA, Ramanna S, Nelson MT, Ugurbil K, and Yacoub E, “Breast Diffusion Weighted Imaging with Reduced Artifacts using Multi-band Spin Echo EPI. Proc ISMRM, Toronto; 2015:0884.
11. “Quantitative MRI (qMRI) Breast Phantom,” High Precision Devices, Inc. [Online]. Available: http://hpd-online.com/breast-phantom.php.
12. Keenan KE, Wilmes LJ, Aliu SO, Newitt DC, Jones EF, Boss MA, Stupic KF, Russek SE and Hylton NM, Design of a breast phantom for quantitative MRI. J. Magn. Reson. Imaging, 2016, 44: 610–619. doi:10.1002/jmri.25214)
13. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003, 20(2):870–88.
14. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, and Matthews PM, “Advances in functional and structural MR image analysis and implementation as FSL,” NeuroImage. 2004, 23:S208–S219.
15. “TOPUP,” FSL. [Online]. Available: http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TOPUP.
16. Teruel JR, Fjøsne HE, Østlie A, Holland D, Dale AM, Bathen TF, et al. Inhomogeneous static magnetic field-induced distortion correction applied to diffusion weighted MRI of the breast at 3T: Distortion Correction in DWI of the Breast. Magnetic Resonance in Medicine. 2015, 74(4):1138–44.
Figures