2107
10 Second Temporal Resolution of Early Enhancement Visualization: Framework for Fast Breast MRI Screening1Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States, 3Carbone Cancer Center, University of Wisconsin-Madison, Madison, WI, United States, 4Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 5Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States, 6Department of Emergency Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States
Synopsis
In this work, we present a new methodology for an abbreviated dynamic contrast enhanced (DCE) MRI breast screening protocol. The methodology relays on a novel dynamic reconstruction scheme, local low-rank, applied to breast MR imaging for the first time. Our work provides the framework for a high resolution (0.83 mm isotropic), ultra-fast (10 second volumetric frame rate) imaging technology to deliver detailed information of the early enhancement phase of breast lesions, preserving diagnostic accuracy while shortening exam times. We demonstrate the feasibility of the proposed approach.
Introduction
There is substantial interest in shortening screening breast MRI protocols to improve patient access and patient experience, and decrease cost, while preserving diagnostic accuracy1. The purpose of this work was to develop and demonstrate the feasibility of a novel dynamic contrast enhanced (DCE) breast MRI technique that provides 1) sub-millimeter isotropic spatial resolution for lesion morphologic assessment and 10 second (s) temporal resolution for evaluation of early tumor perfusion and 2) a static, fat-water separated, contrast-enhanced breast architecture reconstruction, in six minutes of acquisition time.Methods
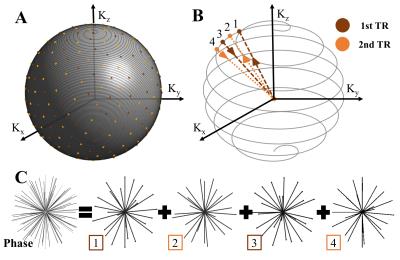
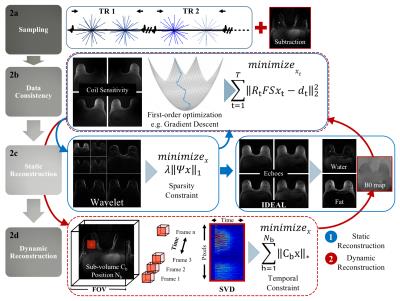
A T1-weighted 3D radial trajectory2 with four unique echoes was used to acquire two, three minute (min) phases (one pre- and one post-contrast) (Figs. 1 and 2a). Two iterative regularization algorithms, marked as dynamic and static reconstructions in Fig. 2, were used as follows:
1) Dynamic reconstruction (Fig. 2d): Pre-contrast data (three min) was subtracted from post-contrast data (three min) in k-space to increase sparsity, then reconstructed using local low-rank (LLR)3 to create a temporal constraint, achieving a 10 s volumetric frame rate with 0.83 mm isotropic spatial resolution.
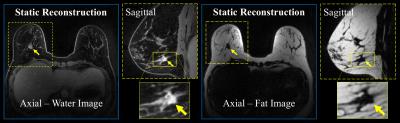
2) Static reconstruction (Fig. 2c): A companion, fat-water separated, breast architecture map is provided by reconstructing the unsubtracted enhanced data using compressed sensing (CS) and Iterative Decomposition with Echo Asymmetry and Least squares estimation (IDEAL)4.
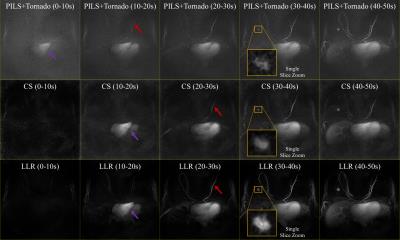
Both reconstructions exploit the 3D radial trajectory to fulfill regularization requirements. Two patients consented to undergo a research breast DCE MRI for this IRB-approved, HIPAA compliant study using a 16-channel breast coil at 3T (Discovery 750, GE Healthcare) over a 32 cm field-of-view (FOV). The proposed method was compared to a CS reconstruction with a strict, 10 s temporal footprint to demonstrate the SNR benefits of the LLR approach while maintaining temporal fidelity. Additionally, our method was compared to a Parallel Imaging reconstruction (PILS)5 using view sharing through a tornado filter that expands its temporal footprint from 30s at the k-space center to 90s temporal at the k-space edge (PILS+Tornado)6.
Results
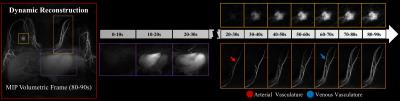
The performance of the dynamic non-linear, local low-rank approach is compared with the linear, spatially invariant view-sharing tornado filter strategy (Fig. 3). The temporal performance with a 10 s volumetric frame rate demonstrated proper visualization of expected physiology, with sequential filling of the right ventricle, left ventricle, mammary arteries and mammary veins (Figs. 3 and 4) despite significant data undersampling (45.5x acceleration). Morphologic details of a known breast cancer, including spiculated margins and an irregular shape, were well-depicted with high spatial resolution (Fig. 4). Rapid acquisition, robust fat suppression, and 0.83 mm isotropic spatial resolution were also achieved in the static reconstruction (Fig. 5).Discussion
We developed an ultra-fast volumetric bilateral breast MRI exam with sub-millimeter isotropic resolution, surpassing the clinical standard while providing temporal resolutions three-to-six fold faster than typical clinical protocols. The dynamic reconstruction approach learns spatially varying models of temporal behaviors through a LLR constraint that effectively permits the temporal footprint to widen in regions of low temporal bandwidth while narrowing in regions where signal intensity is changing rapidly. Widening the temporal footprint in temporally sparse regions provides the SNR for high temporal performance in regions of dynamic signal changes. We hypothesize that the “loss” of the delayed dynamic contrast enhancement pattern through abbreviating a full protocol could be balanced by detailed evaluation of the initial enhancement phase, potentially providing similar or more valuable information than the current methodology with a much shorter scan time7,8. By simultaneously providing high spatial and temporal information of the early enhancement phase, we will provide clinicians and researchers with the capability to extract all the diagnostic and lesion characterization data possible from the early enhancement phase. Further validation to demonstrate the feasibility of our high performance sequence for abbreviated breast MRI is required.Conclusion
We demonstrated a 3D radial breast MRI data acquisition with a dual (dynamic and static) reconstruction methodology to provide 1) 10 s temporal resolution with 0.83 mm isotropic spatial resolution-contrast and 2) a static, post-contrast, fat/water separated breast architecture map over a FOV large enough to image both breasts simultaneously. This combination allows assessment of lesion morphology and early-phase perfusion in a total scan time of six minutes. Upon further validation, this technique may translate to high performance, rapid breast cancer screening with MRI.Acknowledgements
Research supported by NIH R25 GM083252, K24 DK102595 and T32CA009206, RSNA Research & Education Foundation, the Department of Radiology R & D Fund at the Authors’ Institution, Wisconsin Women's Health Foundation, the Science and Medicine Graduate Research Scholars and GE Healthcare.References
1. Kuhl, C. K., Schrading, S., Strobel, K., Schild, H. H., Hilgers, R. D., & Bieling, H. B. (2014). Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol, 32(22), 2304-2310. doi:10.1200/JCO.2013.52.5386
2. Moran, C. J., Brodsky, E. K., Bancroft, L. H., Reeder, S. B., Yu, H., Kijowski, R., . . . Block, W. F. (2014). High-resolution 3D radial bSSFP with IDEAL. Magn Reson Med, 71(1), 95-104. doi:10.1002/mrm.24633
3. Otazo, R., Candes, E., & Sodickson, D. K. (2015). Low-rank plus sparse matrix decomposition for accelerated dynamic MRI with separation of background and dynamic components. Magn Reson Med, 73(3), 1125-1136. doi:10.1002/mrm.25240
4. Reeder, S. B., Pineda, A. R., Wen, Z., Shimakawa, A., Yu, H., Brittain, J. H., . . . Pelc, N. J. (2005). Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med, 54(3), 636-644. doi:10.1002/mrm.20624
5. Griswold, M. A., Jakob, P. M., Nittka, M., Goldfarb, J. W., & Haase, A. (2000). Partially parallel imaging with localized sensitivities (PILS). Magn Reson Med, 44(4), 602-609.
6. Barger, A. V., Block, W. F., Toropov, Y., Grist, T. M., & Mistretta, C. A. (2002). Time-resolved contrast-enhanced imaging with isotropic resolution and broad coverage using an undersampled 3D projection trajectory. Magn Reson Med, 48(2), 297-305. doi:10.1002/mrm.10212
7. Abe, H., Mori, N., Tsuchiya, K., Kulkarni, K., Sheth, D., Schacht, D., . . . Karczmar, G. (2015, November 29 - December 4). Kinetic Analysis of the Ultra Early Phase on Breast MRI: Comparison between Benign and Malignant Lesions using Ultrafast Dynamic Contrast Enhanced MRI. Paper presented at the Radiological Society of North America 2015 Scientific Assembly and Annual Meeting, Chicago IL.
8. Pineda, F., Tsuchiya, K., Abe, H., Medved, M., Wang, S., Fan, X., . . . Karczmar, G. (2015, November 29 - December 4, 2015). Characterization of Breast Lesion Kinetics with Accelerated DCE-MRI Using Conventional Sampling Methods. Paper presented at the Radiological Society of North America 2015 Scientific Assembly and Annual Meeting, Chicago IL.
Figures