2102
Conductivity imaging for assessing the treatment outcome of MR-HIFU ablation of uterine fibroids1University Hospital Cologne, Cologne, Germany, 2Philips Research Europe, Hamburg, Germany, 3Samsung Medical Center, Seoul, Korea, Republic of
Synopsis
MR-HIFU is a non-invasive thermal therapy used to treat symptomatic uterine fibroids. During therapies, clinicians utilize information provided by MRI for treatment planning and to ensure ablation of fibroids using non-invasive temperature monitoring via PRFS thermometry. Thereafter, the therapeutic outcome is determined by measuring the non-perfused volume (NPV) following contrast agent administration. We present a case study using Electric Properties Tomography (EPT) for assessment of treatment outcome by correlating the change in conductivity to the NPV. An increase in conductivity of up to 20% was observed. Thus, EPT is a promising approach for assessment of treatment outcome.
Introduction
Magnetic resonance imaging (MRI)-guided high intensity focused ultrasound (MR-HIFU) is a non-invasive thermal therapy, which is used to treat symptomatic uterine fibroids. During therapies, clinicians utilize the information provided by MRI to plan the treatment, and to ensure ablation (> 56°C) of the fibroids using non-invasive temperature monitoring based on proton resonance frequency shift (PRFS) thermometry. Thereafter, the therapeutic outcome is determined by measuring the non-perfused volume (NPV) following an MR contrast agent (CA) administration. However, there are a few drawbacks associated with using the NPV as a measure for therapeutic outcome. Firstly, presence of gadolinium (Gd)-based MR CA has been shown to influence PRFS thermometry leading to temperature errors between -4 and +3 °C [1]. Consequently, CA is only injected at the end of the therapy, and clinicians do not have the option to immediately perform additional treatment to improve treatment outcome. Secondly, patients who have contra-indication for MR CA are excluded from MR-HIFU. To overcome these limitations, different MRI sequences, which provide comparable information to contrast-enhanced MRI, should be investigated. Here, we present a case study on the use of MR Electric Properties Tomography (EPT, [2]) for assessment of treatment outcome by correlating the change in conductivity to the NPV. An increase in conductivity was expected as ablation causes protein denaturation and cell membrane damage, leading to an increase in ion mobility and concentration, and thus, in conductivity.Materials and Methods
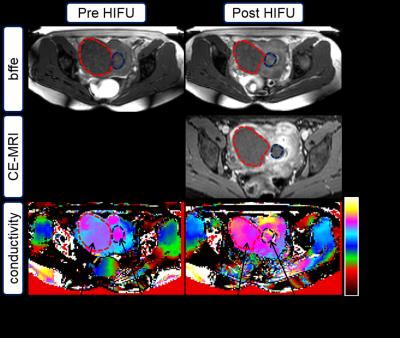
A 37-year-old female patient with multiple fibroids was treated with a volumetric 1.5 T MR-HIFU system (Philips Sonalleve®). EPT was based on a balanced Fast Field Echo (bFFE) sequence (TR/TE=2.4/1.2 ms, voxel=2.5×2.5×2.5 mm³, flip=30°) acquired prior to and at 1.5 hours after MR-HIFU ablation. It is assumed that after this time, temperature of treated tissue is back to normal body temperature, and thus conductivity is not impacted by direct thermal effects [3]. Following acquisition of EPT, gadolinium-tetraazacyclododecanetetraacetic acid (0.1 mmol/kg; Dotarem, Guerbet) was administered intravenously, and contrast-enhanced T1-weighted MRI was performed to evaluate the NPV. Conductivity reconstruction was performed using the phase-based approach of EPT [2] and a subsequent bilateral median filter using tissue boundaries delineated from the bFFE magnitude image [4]. The average conductivity was determined by drawing a region of interest around the whole index fibroid, and subsequently analyzed using ImageJ® [5].Results
As shown in Figure 1, the average conductivities of the subserosal fibroid before and after treatment were 1.02 S/m and 1.14 S/m, respectively. Similarly, the submucosal fibroid showed a 20.9% increase in conductivity from 1.10 S/m before to 1.33 S/m post treatment.Conclusion
EPT is a promising approach for assessment of treatment outcome. Further studies are needed to demonstrate its clinical applicability as EPT has the potential to be used not only as readout at the end of the therapy, but also during the therapy for immediate optimization of treatment approach.Acknowledgements
This research is supported by the EU FP7 grant IPaCT, project ID 603028.
References
[1] Hijnen NM et al. JTU, 2013, 1:8.
[2] Voigt T et al., MRM 66 (2011) 456
[3] Leussler C et al., ISMRM 20 (2012) 3451
[4] Katscher U et al., ISMRM 20 (2012) 3482
[5] Rasband, W.S., ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997-2016.