2093
Test-Retest Reliability of in bore MRI Guided Prostate Biopsy: a pilot study to optimize the current repeated biopsy paradigm in patients on Active Surveillance?1Radiology, Emory University-School of Medicine, Atlanta, GA, United States, 2Urology, Emory University-School of Medicine, Atlanta, GA, United States, 3Pathology, Emory University-School of Medicine, Atlanta, GA, United States
Synopsis
Consistent results throughout repeated biopsy sessions is an essential requirement for any tool used for active surveillance. TRUS biopsy showed inconsistent results in repeated biopsy sessions. Regarding MRI guided biopsy, the reliability of repeated biopsies needs to be established. 5 patients with 17 lesions which were repeatedly biopsied under direct MRI guidance were included. Kappa statistics showed moderate agreement. Negative predictive value for 2nd biopsy was 93% and for 3rd biopsy was 90%. Consistent biopsy results may obviate the need for the current paradigm of obtaining annual prostate biopsies in patients undergoing active surveillance
Introduction and Purpose :
Active surveillance (AS) is offered to patients with low risk prostate cancer to avoid un-necessary morbidity associated with radical treatments. Obtaining consistent results from repeated biopsy sessions is an essential feature for a successful active surveillance sampling tool. TRUS-Bxs are inherently imprecise 1. They show wide variation in both Gleason scores (GS) and tumor volume 1, 2 with overall mild to moderate agreement across repeated sessions 3. In bore MRI guided biopsy may be suitable for active surveillance due to proven targeting precision at both experimental and clinical settings 4, 5. No data were available about the performance of in-bore transrectal MRI guided biopsy (iMRGB) in targeting the same tumor foci by repeated biopsies. We performed a pilot study to evaluate the test-retest reliability of repeated in bore MRI guided biopsy among patients considered candidates for active surveillance as a step to optimize the current paradigm of repeated biopsy.Methods:
We retrospectively analyzed 17 lesions in 5 patients who had consequent iMRGBs from 2013 to 2016. Three patients had only 2 consequent iMRGBs and two patients had 3 consequent biopsy sessions. For each iMRGB, a multiparametric MRI (mpMRI) was performed at different time prior to the iMRGB session using a 3T MRI scanner (Magnetom Trio, Siemens, Germany) with 32-channel surface pelvic coil with our institutional protocol incorporating T2WI, DWI, and DCE. All lesions were diagnosed, localized and biopsied by a single experienced radiologist (18 years of experience). Biopsies were performed on the same MRI scanner using Dyna-TRIM (Invivo, Gainesville, FL) guidance system. During the biopsy session, lesion localization was guided by DWI and T2WI with correlation to the prior diagnostic mpMRI. Samples were labeled with the same identifying number on the mpMRI, biopsy, and histopathology reports. Pathology results were categorized into benign (including inflammation), suspicious lesions (prostatic intraepithelial neoplasia) and malignant (low: GS ≤6, intermediate: GS 7, or high risk :GS ≥ 8). Discrepancies in any of these 3 parameters disqualified the lesions as matching ones. PI-RADS v2 score was assigned to each lesion in retrospect. Consistency between 1st and subsequent biopsy results were analyzed using Kappa statistics. The negative predictive value for prostate cancer was calculated.Results:
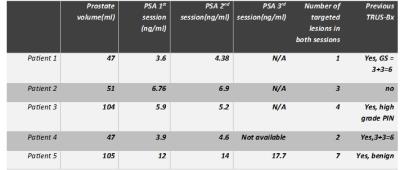
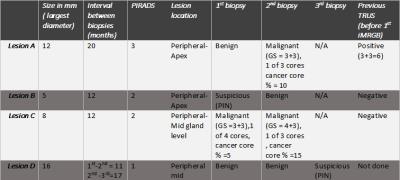
5 patients on active surveillance were included in this study, table 1. Lesions biopsied in 2 consequent sessions: A total of 17 lesions were assessed in this study. 14 lesions (82.4%) had matching 2nd biopsy and 3 (17.6%) had non matching 2nd biopsy. As for lesion locations, most of the matching lesions were in the central gland (11/14, 78.6%) at mid gland levels (9/14, 64 %) and all non-matching lesions were at the peripheral zone (3/3, 100%), mostly at the apex (2/3,66.7%), showing statistically significant difference (p value= 0.029). The mean interval duration for re-biopsy was 9.8±4.9 months for matching lesions vs 14.6±4.6 months for non-matching lesions (p = 0.14). Lesions with both PIRADS categories 1 and 4 were all benign and had matching pathology results. Lesions with non-matching results had PIRADS categories 2 and 3, as illustrated in figure 1. The negative predictive value for prostate cancer in 2nd biopsies was about 93%. Overall agreement was 82.4% and overall disagreement was 17.6%. Positive agreement between 1st and 2nd biopsy sessions was 80% and Negative agreement was 89.6%. Kappa statistics was used to assess the agreement between 1st and 2nd iMRGBs as corrected for chance, Kappa value = 0.55 denoting moderate agreement beyond chance (P value = 0.001). Lesions biopsied in 3 consequent sessions: 10 out of the 17 lesions had a 3rd biopsy session. 9/10 (90%) had matching pathology results across the 3 biopsy sessions and 1/10 (10%) was not matching in the 3rd time. All matching lesions were benign. The negative predictive value for 3rd biopsy (as referenced to 2nd biopsy) was 90%. The non-matching lesion characteristic is illustrated on table 2.Discussion and Conclusion:
This pilot study indicates that in-bore MRI guided biopsy may have a better reliability for repeat biopsies compared to TRUS biopsy. A reliable demonstration of consistent biopsy results may obviate the need for the current paradigm of obtaining annual prostate biopsies in patients undergoing active surveillance. The final conclusion awaits a prospective analysis on a larger cohort of patients.Acknowledgements
No acknowledgement found.References
1. Porten SP, Whitson JM, Cowan JE, Cooperberg MR, Shinohara K, Perez N, et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol. 2011;29(20):2795-800.
2. Porten SP, Whitson JM, Cowan JE, Perez N, Shinohara K, Carroll PR. Changes in cancer volume in serial biopsies of men on active surveillance for early stage prostate cancer. J Urol. 2011;186(5):1825-9.
3. Serefoglu EC, Altinova S, Ugras NS, Akincioglu E, Asil E, Balbay MD. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Canadian Urological Association Journal. 2013;7(5-6):E293-E8.
4. Tokuda J, Tuncali K, Iordachita I, Song SE, Fedorov A, Oguro S, et al. In-bore setup and software for 3T MRI-guided transperineal prostate biopsy. Phys Med Biol. 2012;57(18):5823-40.
5. Seifabadi R, Cho NB, Song SE, Tokuda J, Hata N, Tempany CM, et al. Accuracy study of a robotic system for MRI-guided prostate needle placement. Int J Med Robot. 2013;9(3):305-16.