2081
Improved lymph node staging using MRI mDixon fat fraction measurements in patients with intermediate and high-risk prostate cancer1UCL Centre for Medical Imaging, London, United Kingdom, 2Medical Physics, University College London Hospital, London, United Kingdom, 3Institute of Nuclear Medicine, University College London Hospital, London, United Kingdom
Synopsis
The staging of lymph nodes in prostate cancer is important for the planning and monitoring of treatments. However, there is currently a paucity of techniques that can accurately identify the presence of metastases in small nodes.
In this study, we investigate the usefulness of signal fat fraction from MRI mDixon acquisitions in discriminating between benign and metastatic nodes using 18F-Choline PET as a reference standard.
We present data suggesting that in comparison to commonly used diameter measurements, mDixon fat fraction may be better at discriminating benign from involved lymph nodes that are <10mm in short axis diameter.
Purpose
The spread of adenocarcinoma outside of the prostate to pelvic lymph nodes confers a poorer prognosis in prostate cancer. Indeed, the five-year survival rate in node-positive cases falls threefold when the occurrence of nodal metastases rises from a single instance to >5[1]. Furthermore, the morbidity and mortality associated with surgical lymph node dissection for all patients[1] highlights a need for the development of non-invasive staging techniques. A common convention is to use a size threshold of <10mm in short axis diameter (SAD) of nodes measured on axial MRI or CT to indicate normality [1][2]. This size criterion has a low sensitivity due to the frequent occurrence of metastases in smaller nodes[2]. Alternatively, a ratio of long-to-short node diameter can provide a measure of shape and has been found to decrease in disease[3]. 18F-Choline and prostate-specific membrane antigen PET radiotracers have also shown promise in nodal staging studies with increased uptake observed in metastases[4]. However, these PET techniques require validation and given the expense and limited availability, development of MRI based methods to improve diagnostic accuracy in nodes with SAD <10mm are highly desirable. In addition to other qualitative observations, the presence of a fatty hilum is known to be indicative of benignity[3]. In this work, we assess for the first time whether mDixon quantitative signal fat fraction measurements in pelvic lymph nodes can be used to better characterise nodes with SAD <10mm using 18F-Choline PET as a reference standard.Methods
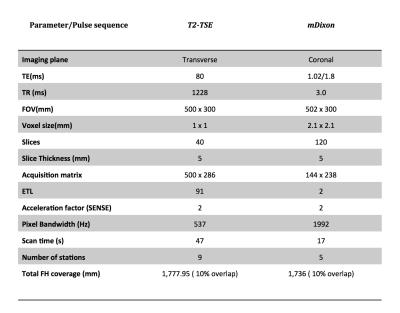
Analysis of mDixon MRI data was conducted in the pelvic lymph nodes of five men with nodal metastases evident on 18F-Choline PET scans. The 18F-Choline PET-CT imaging was performed using an integrated 64-slice PET/CT scanner (Discovery VCT; GE Healthcare) from vertex to mid thigh. mDixon and T2W imaging data was acquired as part of a multiparametric whole body MRI protocol performed using a 3T scanner (Ingenia, Philips Healthcare, Netherlands) from vertex to feet. Parameters are provided in Figure 1.
PET-CT was reported as per standard clinical practise and a board certified radiologist identified the reported PET-positive (n=14) and negative (n=14) nodes on the T2W images. Short and long axis diameter measurements where carried out using the axial T2W images and nodes were segmented on T1W mDixon images for signal fat fraction (FF) quantification. FF was calculated from ROI signal intensity (SI) as SIfat / (SIwater + SIfat). Group comparisons were performed between PET-positive and PET-negative nodes in those with a SAD <10mm. Differences for SAD, long-to-short axis ratio, and FF were compared between node positive and negative cases using two tailed t-tests (Graphpad Prism, v6.01).
Results
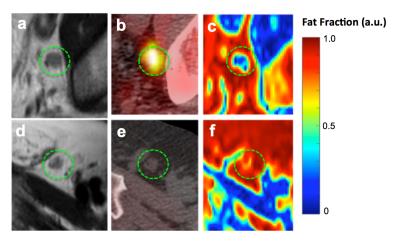
Five of the PET-positive nodes measured >10mm in SAD reducing group size to n=9 (no PET-negative nodes excluded based on size). Axial imaging slices displaying T2W, fused PET-CT, and FF maps in representative PET-positive and PET-negative nodes are displayed in Figure 2. The avid choline uptake observed in Figure 2b suggests involvement (PET-positive), and is not evident on the PET-negative node in Figure 2e (PET-negative). Similarly, FF maps show a qualitative difference between these nodes, with reduced FF signal seen within the PET-positive node (Figure 2c) relative to the PET-negative node (Figure 2f). Quantitatively, a significant reduction in FF was observed for PET-positive vs. negative nodes (P<0.01) (Figure 3c). In comparison, both nodes appear hypointense on T2W images and conventional axis measurements showed no significant difference in either SAD or short–to-long axis ratio between the two groups (Figure 3a,b).Discussion
Signal fat fraction was calculated in pelvic lymph nodes using two point mDixon imaging data, and showed significant differences between node positive and negative disease using a 18F-Choline PET reference standard. The observed reduction in signal fat fraction in the PET-positive nodes likely stems from loss of the fatty hilum caused by metastatic disease. No differences were observed in SAD measurements, consistent with previous studies reporting poor specificity when a SAD threshold of 10mm is used [3]. In future work we aim to address some of the limitations of this preliminary study by increasing our cohort size and by including patients with no identified PET-positive nodes. Scan-rescan repeatability of the fat fraction measurement will also be assessed.Conclusions
Fat fraction, as calculated from mDixon MRI acquisitions has shown promise as a quantitative biomarker to discriminate involved from benign lymph nodes in patients with intermediate and high-risk prostate cancer.Acknowledgements
This research was supported by grants from the Comprehensive Cancer Imaging Centre and the Biomedical Research Centre. The work of JO was supported by a grant from the Mitchell Charitable Trust.References
[1] S. Sankineni, A. M. Brown, M. Fascelli, Y. M. Law, P. A. Pinto, P. L. Choyke, and B. Turkbey, “Lymph node staging in prostate cancer.,” Curr. Urol. Rep., vol. 16, no. 5, p. 30, May 2015.
[2] S. Ganeshalingam and D.-M. Koh, “Nodal staging.,” Cancer Imaging, vol. 9, no. 1, pp. 104–11, 2009.
[3] C. J. McMahon, N. M. Rofsky, and I. Pedrosa, “Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging.,” Radiology, vol. 254, no. 1, pp. 31–46, 2010.
[4] B. Kiss, H. C. Thoeny, and U. E. Studer, “Current Status of Lymph Node Imaging in Bladder and Prostate Cancer,” Urology, Elsevier Inc., 01-Dec-2015.
Figures