2062
Why is the Peripheral Zone of the Normal Human Prostate High in ADC Value and T2-Weighted Signal Intensity?1Centre for Medical Imaging, University College London, London, United Kingdom, 2Centre for Medical Image Computing, University College London, London, United Kingdom, 3Research Department for Tissue & Energy, University College London, London, United Kingdom, 4Department of Pathology, University College London Hospital, University Street, United Kingdom, 5Department of Urology, University College London Hospital, London, United Kingdom, 6Francis Crick Institute, University College London, London, United Kingdom, 7Discipline of Medical Radiation Sciences, University of Sydney, Sydney, Australia
Synopsis
The biophysical basis of MRI signal in the normal human prostate is uncertain, whereby the normal peripheral zone has high ADC values and returns high signal on T2-weighted imaging. In this study, we use MRI in combination with quantitative digital histopathology to offer an explanation. Paired scans were performed at 3T on a human prostate, prior to and following prostatectomy and changes in zonal morphology and MRI characteristics were measured. The peripheral zone collapsed and reduced in T2 signal intensity and ADC value ex-vivo. Digital histopathological analysis suggested the peripheral zone stores more ejaculatory fluid than the transition zone.
PURPOSE:
The prostate gland has a high storage and relatively low secretory capacity for the occasional expulsion of fluid during ejaculation1. McNeal’s work into the structure of the human prostate in the 1980s provided us with the modern understanding of prostate anatomy, as is routinely appreciated on multiparametric MRI (mp-MRI). However, such findings were derived from pathological specimens, which - whilst carefully prepared - cannot reflect tissue microstructure in-vivo. For example, glandular architecture of the normal transition zone (TZ) and peripheral zone (PZ) are often indistinguishable with microscopy1, yet the distinction on T2-weighted MRI is clear. The higher signal of the PZ is often attributed to ‘higher water content’2, without convincing empirical evidence.
In this study, we attempt to answer whether this proposition is true using paired in-vivo and ex-vivo imaging examinations and digital histopathological analysis. Our hypothesis is that the glandular lumen of the PZ store more ejaculatory fluid than in the TZ, explaining the clear distinction found on mp-MRI.
METHODS:
In-vivo imaging
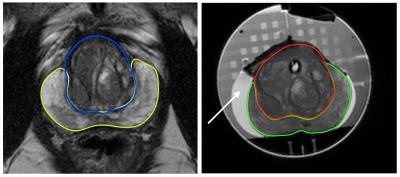
A 55-year-old male enlisted for a prostatectomy for apical Gleason 3+4 cancer underwent ESUR-compliant mp-MRI using a 3 Tesla scanner (Achieva, Philips, NL) prior to surgery. The gland ‘capsule’ was contoured on T2-weighted images by a board certified Radiologist (EJ) using Osirix 7.0 (Bernex, Switzerland) and a patient-specific 3D-printed porous nylon mold created in advance of surgery using these contours to align in-vivo and ex-vivo imaging slices with pathological prostatectomy slices.
Ex-vivo imaging
Following robot assisted laproscopic prostatectomy, the specimen was placed into the mold, immersed in phosphate buffered saline and scanned again at 3T using the same scanner with T2-weighted and diffusion-weighted sequences without formalin fixation.
MR image analysis
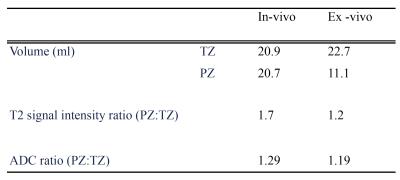
Volume on T2-weighted images and signal intensity ratios of the segmented TZ and PZ volumetrically using T2 and ADC (PZ signal intensity /TZ signal intensity) images were calculated and compared in and ex-vivo (figure 1).
Pathological slide preparation
The specimen was fixed in formalin whilst still in the mold, and the center of the specimen sectioned through slots in the mold to align with MRI slices. The prostatectomy specimen was fixed, sliced and stained with haematoxylin and eosin (H&E) then digitized at 20X magnification using a Nanozoomer digital scanner (Hamamatsu, Japan).
Digital Histopathological analysis
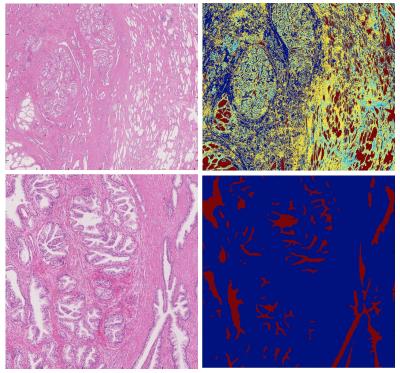
Two tumour-free slices at the mid-gland level and base were selected for the analysis. The entire TZ and PZ were manually segmented on each slice using the freehand region tool on NDP.view (Hamamatsu) (figure 2).
A machine learning technique was used to automatically segment the gland lumen in each manually segmented zone, and quantify and compare surrogate measures of storage capacity. Semi-automatic segmentation was performed using a k-means algorithm modified to account for spatial location of pixels, the simple linear iterative clustering algorithm from the skikit-image module in Python3 (figure 3).
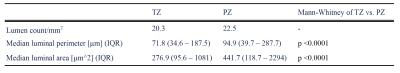
The counts, areas and perimeters per mm2 of the glandular luminal spaces in each zone were calculated and compared using Mann-Whitney U tests.
RESULTS:
Qualitative imaging findings are shown in table 1, and quantitative histology data presented in table 2.DISCUSSION:
Our data supports the hypothesis that the PZ has a higher luminal storage capacity vs. the TZ, and this difference in water content can explain the higher ADC values and T2 signal intensity found in the PZ on mp-MRI. After excision, the PZ collapsed to nearly half its in-vivo size, which is likely to be due to luminal fluid drainage and a loss in glandular exocrine and neuromuscular function following resection. Our hypothesis is also consistent with the decrease in T2 and ADC values as encountered following ejaculation4.
Whilst a loss in gland volume following surgery has been described previously5, we describe for the first time the relative contributions of each zone to this effect, and explained changes in their MR characteristics using a novel image-histological registration pipeline.
CONCLUSION:
The luminal spaces of the peripheral zone have a higher storage capacity for ejaculatory fluid than the transition zone, which explains the biophysical basis of the signal intensity encountered on T2-weighted and ADC images. Further studies to corroborate these findings and consider their implications in the context of cancer would be welcome.Acknowledgements
This work is funded by a grant from Prostate Cancer UK. A grant from the UCLH Biomedical Research Centre supports EJ and SP and EPSRC grant EP/N021967/1 supports EP's work on this topic.References
1 McNeal JE. Normal histology of the prostate. Am J Surg Pathol 1988; 12: 619–33.
2 Turkbey B, Pinto PA, Choyke PL. Imaging techniques for prostate cancer: implications for focal therapy. Nat Rev Urol 2009; 6: 191–203.
3 Achanta R, Shaji A, Smith K et al. SLIC Superpixels Compared to State-of-the-art Superpixel Methods.
4 Medved M, Sammet S, Yousuf A et al. MR Imaging of the Prostate and Adjacent Anatomic Structures before, during, and after Ejaculation: Qualitative and Quantitative Evaluation. Radiology 2014; 271: 452–60.
5 Orczyk C, Taneja SS, Rusinek H et al. Assessment of change in prostate volume and shape following surgical resection through co-registration of in-vivo MRI and fresh specimen ex-vivo MRI. Clin Radiol 2014; 69: e398–403.
Figures