2050
Receiver operating characteristic analysis of fat-fraction is effective in differentiating identifying an optimal threshold to differentiate between brown and white adipose tissue ex vivo and in situ in rats using 3-point IDEAL MRI1Department of Radiology, University Hospitals Coventry & Warwickshire NHS Trust, Birmingham, United Kingdom, 2Warwick Medical School, University of Warwick, Coventry, United Kingdom, 3Department of Endocrinology, University Hospitals Coventry & Warwickshire NHS Trust, Birmingham, United Kingdom, 4Medical Physics, University Hospitals Coventry & Warwickshire NHS Trust, Coventry, United Kingdom
Synopsis
Brown adipose tissue (BAT) has lower fat content than white adipose tissue (WAT), which has been exploited using Dixon-based MRI to identify BAT. We sought to identify the optimal threshold to differentiate between BAT and WAT in rats on the basis of fat fraction.
Fat fraction within BAT was significantly lower than WAT in rodents. Receiver operating characteristic analysis showed that differentiating BAT and WAT on the basis of fat fraction had excellent accuracy in both ex vivo and in situ. The optimal cut-off to separate BAT and WAT was significantly lower in rats exposed to cold, likely secondary to lipolysis.
Purpose
Lower fat content within BAT compared to white adipose tissue (WAT) has been exploited using Dixon-based MRI imaging methods to visualize BAT in rodents1, human infants2 and adults3.
We sought to determine the optimal threshold for discriminating between interscapular BAT and WAT in Wistar rats post mortem on the basis of fat-fraction using 3-point IDEAL MRI
Method
Four male Wistar rats (mean mass 300g, age 6-8 weeks) reared on an ad libitum diet were used. Two were kept warm (30°C) and two cold (4°C) for 8 hours prior to sacrifice. Interscapular BAT, omental and subcutaneous white adipose tissue (WAT) samples were dissected from one of each group.
A 3-point IDEAL 2D FSE sequence was acquired of excised tissues and rat carcasses on a GE Optima MR360 1.5T MRI scanner (General Electric Medical Systems, Milwaukee, USA) using a HD wrist coil, generating water-only and fat-only images, which were post-processed using ImageJ4 to produce fat fraction maps. Scanning parameters were: slice thickness (mm)/TR(ms)/TE(ms)/matrix/NEX/FoV(cm) = 2.7/400/14.2/120 x 120/9/27.6 for excised specimens, and 2.7/303/13.1/120 x 120/6/56.4 for carcasses.
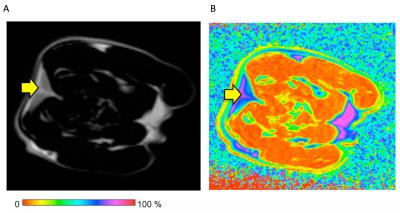
Regions of interest (ROIs) were drawn around the interscapular fat pad (the most readily identifiable BAT deposit in rodents) on the fat-only IDEAL images. ROIs were manually defined within WAT and both transposed onto the fat fraction maps.
Fat fraction within interscapular BAT, omental and subcutaneous WAT ROIs were compared using the Kruskal-Wallis one way analysis of variance. Receiver operating characteristic (ROC) analyses were performed, and the optimal threshold for separating BAT and WAT identified using Youden’s J statistic.
Results
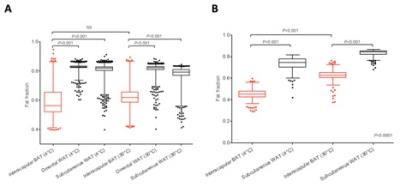
Mean fat fraction within ex vivo interscapular BAT (58.9 ± 10.1% and 62.4 ± 8.6%, at 4°C and 30°C respectively) was significantly lower (p<0.001) than both omental (82.5 ± 2.0% and 81.4 ± 3.1%) and subcutaneous WAT (80.7 ± 4.2 and 78.0 ± 4.0) at both 4°C and 30°C (Figure 2A).
Mean fat fraction within in situ interscapular BAT (44.7 ± 5.4% at 4°C, and 62.2 ± 6.5% at 30°C) was also significantly lower (p<0.001) than subcutaneous WAT (72.9 ± 7.3% at 4°C, and 83.0 ± 3.5% at 30°C, p<0.001), although BAT fat fraction within the rat kept at 4°C was significantly lower than at 30°C (Figure 2B).
ROC analysis of the dissected ex vivo BAT and WAT specimens showed excellent tissue separation (area under the curve, AUC 0.928). The optimal cut-off was a fat fraction of 73.6%, yielding a sensitivity of 86.7% and specificity of 88.1%.
Fat fraction proved very effective in separating BAT and WAT in situ (AUC 0.998 at 4°C, and 0.997 at 30°C). The optimal cut-off point to differentiate BAT and WAT was 75.8% fat fraction for the rat housed at 30°C (sensitivity 99.3%, specificity 94.9%), and 57.8% (sensitivity 99.4%, specificity 96.7%) for the rat housed at 4°C.
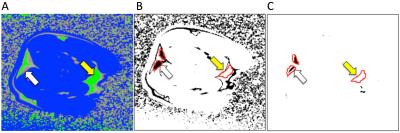
Applying the thresholds to fat fraction maps generated excellent tissue discrimination, which was refined by applying an edge-erode algorithm to remove tissue interfaces (Figure 3).
Discussion
Our results confirm that interscapular BAT within rats has a significantly lower fat fraction than WAT both ex vivo and in situ, which is in line with other studies1. Segmentation on the basis of fat fraction is able to distinguish murine interscapular BAT and WAT with high accuracy.
We also identified that fat fraction within in situ interscapular BAT was lower in those rats exposed to cold than those kept warm, which accords with results from other studies5. This difference was not evident in ev vivo samples, possibly as a result of tissue handling. It is probable that BAT was active in the rats housed at 4°C, and that intracellular lipid stores were depleted to provide substrate6.
It has been suggested that MRI has the potential to identify BAT irrespective of its activation state3. Fat fraction varies in response to the activation state of BAT, therefore segmentation using fat fraction may need to be adjusted in light of the metabolic state of BAT.
Conclusion
Fat fraction proved very effective in distinguishing interscapular BAT and WAT in rats both ex vivo and in situ, but the optimum fat fraction cut-off was significantly lower in presumed metabolically active in situ BAT.Acknowledgements
Thanks to Mr Eddie Ng’andwe, Research Radiographer.References
(1) Hu, H.H., et al., Identification of brown adipose tissue in mice with fat-water IDEAL-MRI. J Magn Reson Imaging, 2010. 31(5): 1195-202.
(2) Hu, H.H., et al., Unequivocal identification of brown adipose tissue in a human infant. J Magn Reson Imaging, 2012. 35(4): 938-42.
(3) Holstila, M., et al., Measurement of brown adipose tissue mass using a novel dual-echo magnetic resonance imaging approach: a validation study. Metabolism, 2013. 62(8): 1189-98.
(4) Schindelin, J., et al., Fiji: an open-source platform for biological-image analysis. Nat Methods, 2012. 9(7): 676-82.
(5) Smith, D.L., Jr., et al., Measurement of interscapular brown adipose tissue of mice in differentially housed temperatures by chemical-shift-encoded water-fat MRI. J Magn Reson Imaging, 2013. 38(6): 1425-33.
(6) Baba, S., et al., CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. J Nucl Med, 2010. 51(2): 246-50.
Figures