2013
Value of Noncontrast MR Imaging with Diffusion-weighted Imaging for Detection of Primary Small (≤ 20mm) Solid Pancreatic Tumors and Prediction of Pancreas Ductal Adenocarcinoma1Radiology, Chung-Ang Universitiy Hospital, Seoul, Korea, Republic of, 2Samsung medical center
Synopsis
With recent advances of MRI in abdominal imaging, improved performances on T2-weighted image and diffusion- weighted imaging (DWI) are achieved for pancreatic tumors. We hypothesized that diagnostic performance for detection of primary solid tumors of pancreas on noncontrast MRI with DWI could be sufficiently high and noncontrast MRI with DWI would be useful for pancreas screening. We conducted this study to determine the diagnostic performance of noncontrast MRI with DWI for detection of primary small (≤20mm) pancreatic solid tumors and prediction of ductal adenocarcinoma in comparison with pancreas CT and pancreas MRI with MR cholangiopancreatography.
Purpose
To determine the diagnostic performance of noncontrast MRI with diffusion-weighted imaging (DWI) (NonMRI) for detection of primary small (≤ 20mm) pancreatic solid tumor and prediction of pancreas ductal adenocarcinoma (PDAC) in comparison with pancreas CT (PanCT) and pancreas MRI with MR cholangiopancreatography (MRCP) (PanMRI)Methods
The institutional review board approved this retrospective study and waived the requirement for informed consent. A total of 126 patients who underwent PanCT and PanMRI, including 94 small (≤ 20mm) pancreatic tumors (51 PDACs, 34 neuroendocrine tumors [NETs], 9 solid pseudopapillary tumors [SPTs]), and 32 patients with normal pancreas, comprised study population.
Two observers assessed three sets of images: PanCT, PanMRI (T1- and T2-weighted images [T1, T2WI], gadoxetic acid-enhanced imaging, MRCP and DWI), and NonMRI (T1- , T2WI and DWI).
The readers reviewed these data sets for the presence of solid pancreatic tumor and likelihood of PDAC. The confidence level for presence of solid pancreatic tumor was rated using a following four-point score (detection score): 1, definitely absent; 2, possibly present; 3, probably present; 4, definitely present. Then, the readers graded the likelihood of PDAC using a following four-point score (PDAC score): 1, no pancreatic tumor; 2, probable non-PDAC tumor; 3, probable PDAC; 4, definite PDAC.
Receiver operating characteristic curve analysis, diagnostic accuracy (area under the receiver operating characteristic curve [Az]) were used for statistical analysis.
Results
Both observers interpreted all of the 32 cases of control group as definitely absent solid pancreatic tumor on all three of the imaging sets. For detection of 94 tumors, NonMRI and PanMRI showed significantly higher detection score than those of PanCT (91.5% [86/94]) for both observer (P < 0.01), and all of 51 PDACs were rated as possibly present to definitely present solid lesions by both observers. And, there was no significant difference between NonMRI and PanMRI for tumor detection for both observers (P = 1.00, P = 1.00, respectively). On PanCT, both observers did not detect eight tumors (6 NETs, 1 PDAC, 1 SPT), but did all of those tumors on PanMRI and NonMRI (P < 0.01).
On ROC analysis of confidence score for the likelihood of PDAC, the Az value of the NonMRI (0.884 for observer 1; 0.930 for observer 2) was comparable with PanCT (0.922 for observer 1; 0.924 for observer 2) for both observers (P > 0.05). Although, statistically significant difference was observed between the Az value of the NonMRI and PanMRI for both observers (0.930 for observer 1; 0.977 for observer 2 on PanMRI) (P < 0.05), but all of 51 PDACs were considered as probable or definite PDAC (PDAC score 3 or 4) on NonMRI by both observers. On Pan CT, one PDAC was considered as probable non-PDAC tumor (PDAC score 2) by both observers. With regard to accuracy, sensitivity, specificity, positive predictive value, and negative predictive value for prediction of PDAC on three imaging set, although there were no statistically significant differences, but a trend toward higher sensitivity and negative predictive value was observed with the NonMRI and PanMRI than those with PanCT for both observers.
Discussion
In this study, noncontrast MR imaging with DWI showed a significantly high detection rate for small pancreatic tumors compared to pancreas CT and comparable results with pancreas MR imaging with MRCP. In addition, all of the 51 PDACs were rated as possibly to definitely present solid lesions and considered as probable or definite PDAC, and 33 of 34 (97.0%) NETs and all 9 SPTs were detected by both observers on noncontrast MR imaging with DWI.
The high capability of noncontrast MR imaging with DWI for detection of primary small solid pancreatic tumors in this study may be based on following strengths of MR sequences: hyperintensity of normal pancreatic parenchyma in contrast to hypointensity of pancreatic focal lesions on fat-suppressed T1WI, increased conspicuity of MPD and CBD on reinforced T2WI at 3T-MR imaging, and the high contrast resolution of tumors on DWI mainly due to decreased water diffusion in the tumor caused by high cellularity, tissue disorganization, and increased extracellular tortuosity. In this study, NonMRI showed higher sensitivity than PanCT and the same sensitivity as PanMRI for the prediction of small PDACs from NETs and SPTs. These morphologic features of PDACs may be represented well on the unenhanced T1- and T2WIs even without enhanced MR images.
Conclusion
NonMRI showed better performance than PanCT, and competitive performance to PanMRI for detection of primary small solid pancreatic tumors, and showed reasonable sensitivity for prediction of PDACs compared with PanCT and PanMRI.Acknowledgements
N/AReferences
1. Vasen HF, Wasser M, van Mil A, et al. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology 2011;140(3):850-856.
2. Barral M, Taouli B, Guiu B, et al. Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology 2015;274(1):45-63.
3. Jang KM, Kim SH, Kim YK, et al. Imaging features of small (</= 3 cm) pancreatic solid tumors on gadoxetic-acid-enhanced MR imaging and diffusion-weighted imaging: an initial experience. Magn Reson Imaging 2012;30(7):916-925.
4. Park MJ, Kim YK, Choi SY, Rhim H, Lee WJ, Choi D. Preoperative detection of small pancreatic carcinoma: value of adding diffusion-weighted imaging to conventional MR imaging for improving confidence level. Radiology 2014;273(2):433-443.
5. Ansari NA, Ramalho M, Semelka RC, Buonocore V, Gigli S, Maccioni F. Role of magnetic resonance imaging in the detection and characterization of solid pancreatic nodules: An update. World J Radiol 2015;7(11):361-374.
6. Yoon SH, Lee JM, Cho JY, et al. Small (</= 20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 2011;259(2):442-452.
7. Takakura K, Sumiyama K, Munakata K, et al. Clinical usefulness of diffusion-weighted MR imaging for detection of pancreatic cancer: comparison with enhanced multidetector-row CT. Abdom Imaging 2011;36(4):457-462.
Figures
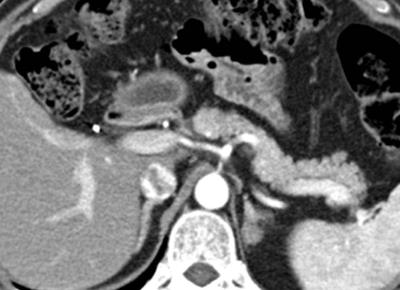
Figure. 1 Pancreatic ductal adenocarcinoma in a 63-year-old man with persistent elevation of CA 19-9. There is no identifiable tumor on the pancreatic phase CT image.
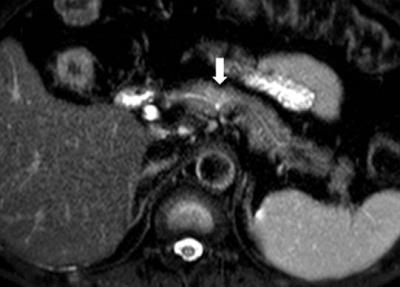
Figure. 2 Pancreatic ductal adenocarcinoma in a 63-year-old man with persistent elevation of CA 19-9.
In pancreas MR imaging, a small ill-defined tumor is identified as a hyperintensity on the T2-weighted image (figure 2), DWI with b= 800 s/mm2 (figure 3), and a hypointensity on the T1-weighted image (figure 4) compared with the normal pancreatic parenchyma. The tumor is less clearly shown on arterial phase image (figure 5).
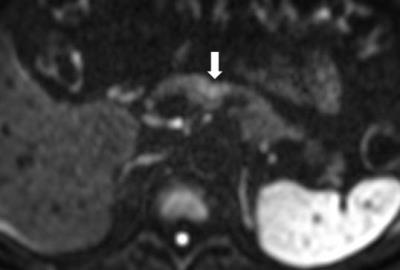
Figure. 3 Pancreatic ductal adenocarcinoma in a 63-year-old man with persistent elevation of CA 19-9.
In pancreas MR imaging, a small ill-defined tumor is identified as a hyperintensity on the T2-weighted image (figure 2), DWI with b= 800 s/mm2 (figure 3), and a hypointensity on the T1-weighted image (figure 4) compared with the normal pancreatic parenchyma. The tumor is less clearly shown on arterial phase image (figure 5).
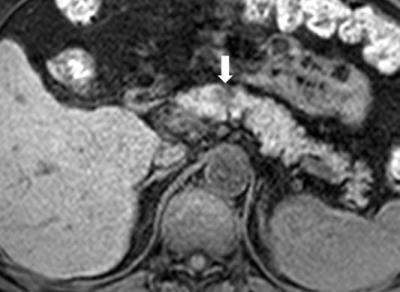
Figure. 4 Pancreatic ductal adenocarcinoma in a 63-year-old man with persistent elevation of CA 19-9.
In pancreas MR imaging, a small ill-defined tumor is identified as a hyperintensity on the T2-weighted image (figure 2), DWI with b= 800 s/mm2 (figure 3), and a hypointensity on the T1-weighted image (figure 4) compared with the normal pancreatic parenchyma. The tumor is less clearly shown on arterial phase image (figure 5).
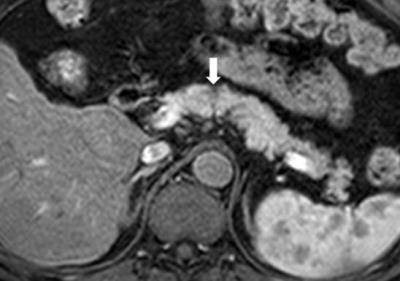
Figure. 5 Pancreatic ductal adenocarcinoma in a 63-year-old man with persistent elevation of CA 19-9.
In pancreas MR imaging, a small ill-defined tumor is identified as a hyperintensity on the T2-weighted image (figure 2), DWI with b= 800 s/mm2 (figure 3), and a hypointensity on the T1-weighted image (figure 4) compared with the normal pancreatic parenchyma. The tumor is less clearly shown on arterial phase image (figure 5).